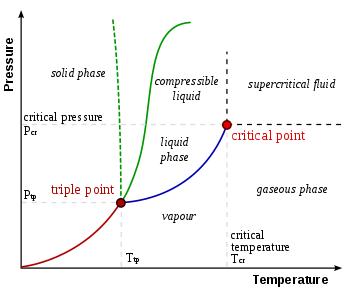
Triple point
Below this, in the vacuum of outer space, solid ice sublimates, transitioning directly into water vapor when heated at a constant pressure.
Conversely, above the triple point, solid ice first melts into liquid water upon heating at a constant pressure, then evaporates or boils to form vapor at a higher temperature.
The melting point of ordinary ice decreases with pressure, as shown by the phase diagram's dashed green line.
Historically, during the Mariner 9 mission to Mars, the triple point pressure of water was used to define "sea level".
For those high-pressure forms of ice which can exist in equilibrium with liquid, the diagram shows that melting points increase with pressure.
For exacting work, triple-point cells are typically filled with a highly pure chemical substance such as hydrogen, argon, mercury, or water (depending on the desired temperature).
Triple-point cells are so effective at achieving highly precise, reproducible temperatures, that an international calibration standard for thermometers called ITS–90 relies upon triple-point cells of hydrogen, neon, oxygen, argon, mercury, and water for delineating six of its defined temperature points.