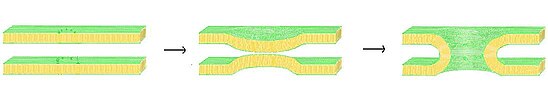
Vesicle fusion
Cardiolipin is found mainly in mitochondrial membranes, and calcium ions play an important role in the respiratory processes mediated by the mitochondrion.
The forces involved have been postulated to explain[3] this process in terms of nucleation for agglomeration of smaller supramolecular entities or phase changes in the structure of the biomembranes.
This closeness allows the cell membrane and the vesicle to exchange lipids which is mediated by certain proteins which remove water that comes between the forming junction.
[7] Assembly of the SNAREs into the "trans" complexes likely bridges the opposing lipid bilayers of membranes belonging to cell and secretory granule, bringing them in proximity and inducing their fusion.
[10] According to the "zipper" hypothesis, the complex assembly starts at the N-terminal parts of SNARE motifs and proceeds towards the C-termini that anchor interacting proteins in membranes.
Based on the stability of the resultant cis-SNARE complex, it has been postulated that energy released during the assembly process serves as a means for overcoming the repulsive forces between the membranes.
There are several models that propose explanation of a subsequent step – the formation of stalk and fusion pore, but the exact nature of these processes remains debated.
[13] There is not currently a proposed mechanism on inter-cellular regulation for fluctuation of lipid-lined pores, and they would have a substantially more difficult time producing effects such as the "kiss-and-run" when compared with their protein-lined counterparts.
Lipid-lined pores effectiveness would also be highly dependent on the composition of both membranes, and its success or failure could vary wildly with changes in elasticity and rigidity.
It has, however, been proven that in vitro Syntaxin per se is sufficient to drive spontaneous calcium independent fusion of synaptic vesicles containing v-SNAREs.