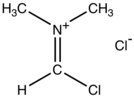
Vilsmeier reagent
The Vilsmeier reagent is an organic compound with the formula [(CH3)2NCHCl]Cl.
It is a salt consisting of the N,N-dimethyliminium cation ([(CH3)2N=CHCl]+) and chloride anion.
Analogues of this particular reagent are generated when tertiary amides other than DMF are treated with POCl3.
The salt is a white solid that is soluble in polar organic solvents.
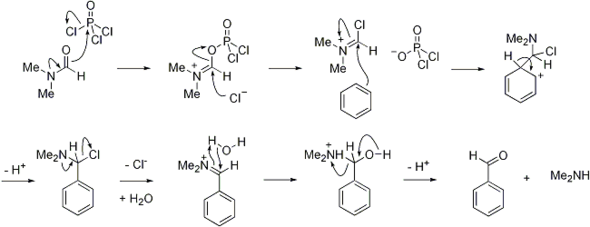
Vilsmeier reagent is the active intermediate in the formylation reactions, the Vilsmeier reaction or Vilsmeier-Haack reaction that use mixtures of dimethylformamide and phosphorus oxychloride to generate the Vilsmeier reagent, which in turn attacks a nucleophilic substrate and eventually hydrolyzes to give formyl.