Virial expansion
The virial expansion is a model of thermodynamic equations of state.
It expresses the pressure P of a gas in local equilibrium as a power series of the density.
This equation may be represented in terms of the compressibility factor, Z, as
This equation was first proposed by Kamerlingh Onnes.
The leading coefficient A is defined as the constant value of 1, which ensures that the equation reduces to the ideal gas expression as the gas density approaches zero.
The second, B, and third, C, virial coefficients have been studied extensively and tabulated for many fluids for more than a century.
Two of the most extensive compilations are in the books by Dymond[2][3] and the National Institute of Standards and Technology's Thermo Data Engine Database[4] and its Web Thermo Tables.
[5] Tables of second and third virial coefficients of many fluids are included in these compilations.
Most equations of state can be reformulated and cast in virial equations to evaluate and compare their implicit second and third virial coefficients.
The seminal van der Waals equation of state[6] was proposed in 1873:
It can be rearranged by expanding 1/(v − b) into a Taylor series:
In the van der Waals equation, the second virial coefficient has roughly the correct behavior, as it decreases monotonically when the temperature is lowered.
Almost all subsequent equations of state are derived from the van der Waals equation, like those from Dieterici,[7] Berthelot,[8] Redlich-Kwong,[9] and Peng-Robinson[10] suffer from the singularity introduced by 1/(v - b).
Other equations of state, started by Beattie and Bridgeman,[11] are more closely related to virial equations, and show to be more accurate in representing behavior of fluids in both gaseous and liquid phases.
[citation needed] The Beattie-Bridgeman equation of state, proposed in 1928,
The Benedict-Webb-Rubin equation of state[12] of 1940 represents better isotherms below the critical temperature:
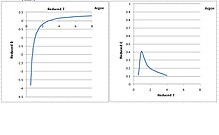
Following are plots of reduced second and third virial coefficients against reduced temperature according to Starling:[13] The exponential terms in the last two equations correct the third virial coefficient so that the isotherms in the liquid phase can be represented correctly.
The exponential term converges rapidly as ρ increases, and if only the first two terms in its Taylor expansion series are taken,
After the expansion of the exponential terms, the Benedict-Webb-Rubin and Starling equations of state have this form:
has the simplicity of the Van der Waals equation of state without its singularity at v = b. Theoretically, the second virial coefficient represents bimolecular attraction forces, and the third virial term represents the repulsive forces among three molecules in close contact.
[citation needed] With this cubic virial equation, the coefficients B and C can be solved in closed form.
the cubic virial equation can be solved to yield:
is therefore 0.333, compared to 0.375 from the Van der Waals equation.
Under the saturation pressure, the liquid phase has a molar volume of
These are the saturation properties needed to compute second and third virial coefficients.
A valid equation of state must produce an isotherm which crosses the horizontal line of
This means that the PρT isotherm has three roots at
In the saturation region, the cubic equation has three roots, and can be written alternatively as:
as a parameter, B and C can be computed in the saturation region of these fluids.
The results are generally in agreement with those computed from Benedict-Webb-Rubin and Starling equations of state.