Electromagnetic absorption by water
The water molecule, in the gaseous state, has three types of transition that can give rise to absorption of electromagnetic radiation: In reality, vibrations of molecules in the gaseous state are accompanied by rotational transitions, giving rise to a vibration-rotation spectrum.
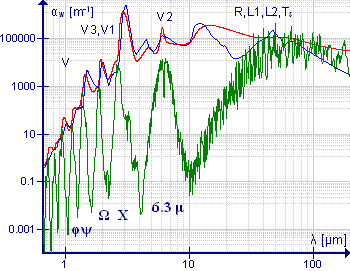
The HITRAN spectroscopy database lists more than 37,000 spectral lines for gaseous H216O, ranging from the microwave region to the visible spectrum.
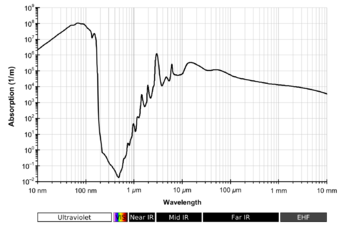
[5][12] In liquid water the rotational transitions are effectively quenched, but absorption bands are affected by hydrogen bonding.
Infrared absorption band positions may be given either in wavelength (usually in micrometers, μm) or wavenumber (usually in reciprocal centimeters, cm−1) scale.
Because of the low symmetry of the molecule, a large number of transitions can be observed in the far infrared region of the spectrum.
Both symmetric stretching and bending vibrations have A1 symmetry, but the frequency difference between them is so large that mixing is effectively zero.
[17] The infrared spectrum of liquid water is dominated by the intense absorption due to the fundamental O-H stretching vibrations.
Because of the high intensity, very short path lengths, usually less than 50 μm, are needed to record the spectra of aqueous solutions.
[14] Direct measurement of the infrared spectra of aqueous solutions requires that the cuvette windows be made of substances such as calcium fluoride which are water-insoluble.
[24] The absorption (equivalent to dielectric loss) is used in microwave ovens to heat food that contains water molecules.
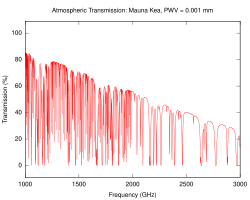
The South Pole Telescope was constructed in Antarctica in part because the elevation and low temperatures there mean there is very little water vapor in the atmosphere.
[26] Similarly, carbon dioxide absorption bands occur around 1400, 1600 and 2000 nm,[27] but its presence in the Earth's atmosphere accounts for just 26% of the greenhouse effect.
[25] Carbon dioxide gas absorbs energy in some small segments of the thermal infrared spectrum that water vapor misses.
[28] In the atmospheric window between approximately 8000 and 14000 nm, in the far-infrared spectrum, carbon dioxide and water absorption is weak.
Water at the top of the troposphere, particularly in liquid and solid states, cools as it emits net photons to space.