Water of crystallization
Water of crystallization can generally be removed by heating a sample but the crystalline properties are often lost.
Per IUPAC's recommendations, the middle dot is not surrounded by spaces when indicating a chemical adduct.
For example, anhydrous RhCl3 is not soluble in water and is relatively useless in organometallic chemistry whereas RhCl3·3H2O is versatile.
Similarly, hydrated AlCl3 is a poor Lewis acid and thus inactive as a catalyst for Friedel-Crafts reactions.
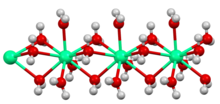
Crystals of hydrated copper(II) sulfate consist of [Cu(H2O)4]2+ centers linked to SO2−4 ions.
Copper is surrounded by six oxygen atoms, provided by two different sulfate groups and four molecules of water.
Water of crystallization is stabilized by electrostatic attractions, consequently hydrates are common for salts that contain +2 and +3 cations as well as −2 anions.
Crystallographic analysis reveals that the solid consists of [trans-NiCl2(H2O)4] subunits that are hydrogen bonded to each other as well as two additional molecules of H2O.
Water is noteworthy because it is reactive, whereas other solvents such as benzene are considered to be chemically innocuous.
Occasionally more than one solvent is found in a crystal, and often the stoichiometry is variable, reflected in the crystallographic concept of "partial occupancy".
It is common and conventional for a chemist to "dry" a sample with a combination of vacuum and heat "to constant weight".
No entries exist for Mo, W, Tc, Ru, Os, Rh, Ir, Pd, Hg, Au.