Wave–particle duality
Wave–particle duality is the concept in quantum mechanics that fundamental entities of the universe, like photons and electrons, exhibit particle or wave properties according to the experimental circumstances.
[1]: 59 It expresses the inability of the classical concepts such as particle or wave to fully describe the behavior of quantum objects.
In the late 17th century, Sir Isaac Newton had advocated that light was corpuscular (particulate), but Christiaan Huygens took an opposing wave description.
[3][4] Thomas Young's interference experiments in 1801, and François Arago's detection of the Poisson spot in 1819, validated Huygens' wave models.
[5] Max Planck heuristically derived a formula for the observed spectrum by assuming that a hypothetical electrically charged oscillator in a cavity that contained black-body radiation could only change its energy in a minimal increment, E, that was proportional to the frequency of its associated electromagnetic wave.
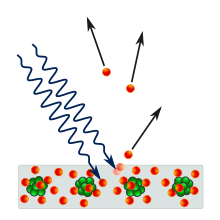
Despite confirmation by various experimental observations, the photon theory (as it came to be called) remained controversial until Arthur Compton performed a series of experiments from 1922 to 1924 demonstrating the momentum of light.
[7]: 211 The experimental evidence of particle-like momentum and energy seemingly contradicted the earlier work demonstrating wave-like interference of light.
[8] In 1924, Louis de Broglie introduced his theory of electron waves in his PhD thesis Recherches sur la théorie des quanta.
[12][13][14][15][16] George Paget Thomson and Alexander Reid at Cambridge University scattered electrons through thin metal films and observed concentric diffraction rings.
[17] Alexander Reid, who was Thomson's graduate student, performed the first experiments,[18] but he died soon after in a motorcycle accident[19] and is rarely mentioned.
Davisson and Thomson were awarded the Nobel Prize in 1937 for experimental verification of wave property of electrons by diffraction experiments.
[21] Similar crystal diffraction experiments were carried out by Otto Stern in the 1930s using beams of helium atoms and hydrogen molecules.
These experiments further verified that wave behavior is not limited to electrons and is a general property of matter on a microscopic scale.
Stars, planets, spacecraft, tennis balls, bullets, sand grains: particle models work across a huge scale.
Having observed wave behavior, now change the experiment, lowering the intensity of the electron source until only one or two are detected per second, appearing as individual particles, dots in the video.
Despite confirmation by various experimental observations, the photon theory (as it came to be called later) remained controversial until Arthur Compton performed a series of experiments from 1922 to 1924 demonstrating the momentum of light.
When these detectors are inserted, quantum mechanics predicts that the interference pattern disappears because the detected part of the electron wave has changed (loss of coherence).
If the laser intensity is turned sufficiently low, individual dots appear on the cameras, building up the pattern as in the electron example.