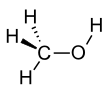
Methanol
Moderately toxic for small animals – Highly toxic to large animals and humans (in high concentrations) – May be fatal/lethal or cause blindness and damage to the liver, kidneys, and heart if swallowed – Toxicity effects from repeated over exposure have an accumulative effect on the central nervous system, especially the optic nerve – Symptoms may be delayed, become severe after 12 to 18 hours, and linger for several days after exposure[9]
[23] In 2006, astronomers using the MERLIN array of radio telescopes at Jodrell Bank Observatory discovered a large cloud of methanol in space 0.463 terametres (288 million miles) across.
[24][25] In 2016, astronomers detected methanol in a planet-forming disc around the young star TW Hydrae using the Atacama Large Millimeter Array radio telescope.
[26] In their embalming process, the ancient Egyptians used a mixture of substances, including methanol, which they obtained from the pyrolysis of wood.
[28] They also introduced the word "methylène" to organic chemistry, forming it from Greek methy = "alcoholic liquid" + hȳlē = "forest, wood, timber, material".
[9] German chemists Alwin Mittasch and Mathias Pier, working for Badische-Anilin & Soda-Fabrik (BASF), developed a means to convert synthesis gas (a mixture of carbon monoxide, carbon dioxide, and hydrogen) into methanol and received a patent.
[30] US patent 1,569,775 was applied for on 4 September 1924 and issued on 12 January 1926 to BASF;[31] the process used a chromium and manganese oxide catalyst with extremely vigorous conditions: pressures ranging from 50 to 220 atm, and temperatures up to 450 °C.
Modern methanol production has been made more efficient through use of catalysts (commonly copper) capable of operating at lower pressures.
The modern low pressure methanol (LPM) process was developed by ICI in the late 1960s with the technology patent long since expired.
[32] During World War II, methanol was used as a fuel in several German military rocket designs, under the name M-Stoff, and in a roughly 50/50 mixture with hydrazine, known as C-Stoff.
Condensation of methanol to produce hydrocarbons and even aromatic systems is the basis of several technologies related to gas to liquids.
[39][40] Methanol is a promising energy carrier because, as a liquid, it is easier to store than hydrogen and natural gas.
It burns forming carbon dioxide and water: Methanol fuel has been proposed for ground transportation.
However, it is equivalent to super high-octane gasoline in horsepower, and most modern computer-controlled fuel injection systems can already use it.
[45] Direct-methanol fuel cells are unique in their low temperature, atmospheric pressure operation, which lets them be greatly miniaturized.
[46][47] This, combined with the relatively easy and safe storage and handling of methanol, may open the possibility of fuel cell-powered consumer electronics, such as laptop computers and mobile phones.
Methanol burns well in an unpressurized burner, so alcohol stoves are often very simple, sometimes little more than a cup to hold fuel.
This was commonly used during the US prohibition to discourage consumption of bootlegged liquor, and ended up causing several deaths.
Today, the most widely used catalyst is a mixture of copper and zinc oxides, supported on alumina, as first used by ICI in 1966.
One way of dealing with the excess hydrogen is to inject carbon dioxide into the methanol synthesis reactor, where it, too, reacts to form methanol according to the equation In terms of mechanism, the process occurs via initial conversion of CO into CO2, which is then hydrogenated:[54] where the H2O byproduct is recycled via the water-gas shift reaction This gives an overall reaction which is the same as listed above.
In a process closely related to methanol production from synthesis gas, a feed of hydrogen and CO2 can be used directly.
[55] The main advantage of this process is that captured CO2 and hydrogen sourced from electrolysis could be used, removing the dependence on fossil fuels.
[64] Another method of producing green methanol involves combining hydrogen, carbon dioxide, and a catalyst under high heat and pressure.
In addition to water, typical impurities include acetone and ethanol (which are very difficult to separate by distillation).
Methanol fires should be extinguished with dry chemical, carbon dioxide, water spray or alcohol-resistant foam.
Ingesting as little as 10 mL (0.34 US fl oz) of pure methanol can cause permanent blindness by destruction of the optic nerve.
[66] The median lethal dose is 100 mL (3.4 US fl oz), i.e., 1–2 mL/kg body weight of pure methanol.
This addition of methanol exempts industrial ethanol (commonly known as "denatured alcohol" or "methylated spirit") from liquor excise taxation in the US and other countries.