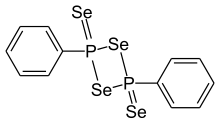
Woollins' reagent
It can also be conveniently prepared in the laboratory by heating a mixture of dichlorophenylphosphine and sodium selenide (Na2Se), (itself prepared from reacting elementary selenium with sodium in liquid ammonia).
[2] An alternative synthesis is the reaction of the pentamer (PPh)5 (pentaphenylcyclopentaphosphine) with elemental selenium.
[3] The main use of Woollins' reagent is the selenation of carbonyl compounds.
[4] For instance, Woollins' reagent will convert a carbonyl into a selenocarbonyl.
Additionally, Woollins' reagent has been used to selenonate carboxylic acids, alkenes, alkynes, and nitriles.