Wound healing
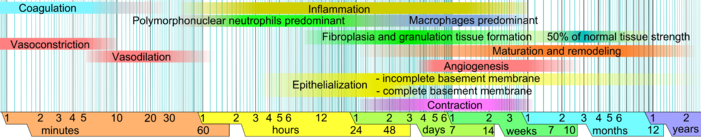
The cellular phase involves several types of cells working together to mount an inflammatory response, synthesize granulation tissue, and restore the epithelial layer.
Platelets, the cells present in the highest numbers shortly after a wound occurs, release mediators into the blood, including cytokines and growth factors.
Platelets release other proinflammatory factors like serotonin, bradykinin, prostaglandins, prostacyclins, thromboxane, and histamine,[3] which serve several purposes, including increasing cell proliferation and migration to the area and causing blood vessels to become dilated and porous.
[3][15] Histamine also causes blood vessels to become porous, allowing the tissue to become edematous because proteins from the bloodstream leak into the extravascular space, which increases its osmolar load and draws water into the area.
[29][30] Attracted to the wound site by growth factors released by platelets and other cells, monocytes from the bloodstream enter the area through blood vessel walls.
[15] In wound healing that result in incomplete repair, scar contraction occurs, bringing varying gradations of structural imperfections, deformities and problems with flexibility.
[33][34] As inflammation dies down, fewer inflammatory factors are secreted, existing ones are broken down, and numbers of neutrophils and macrophages are reduced at the wound site.
[3] Origins of these fibroblasts are thought to be from the adjacent uninjured cutaneous tissue (although new evidence suggests that some are derived from blood-borne, circulating adult stem cells/precursors).
[39] Initially fibroblasts utilize the fibrin cross-linking fibers (well-formed by the end of the inflammatory phase) to migrate across the wound, subsequently adhering to fibronectin.
Growth factors (PDGF, TGF-β) and fibronectin encourage proliferation, migration to the wound bed, and production of ECM molecules by fibroblasts.
[40] Type III collagen and fibronectin generally begin to be produced in appreciable amounts at somewhere between approximately 10 hours[41] and 3 days,[37] depending mainly on wound size.
With the lack of hair follicles, nerves and sweat glands, the wound, and the resulting healing scar, provide a challenge to the body with regards to temperature control.
[52] Growth factors are also important for the innate immune defense of skin wounds by stimulation of the production of antimicrobial peptides and neutrophil chemotactic cytokines in keratinocytes.
[32] The breakdown of the provisional matrix leads to a decrease in hyaluronic acid and an increase in chondroitin sulfate, which gradually triggers fibroblasts to stop migrating and proliferating.
[31] The onset of the maturation phase may vary extensively, depending on the size of the wound and whether it was initially closed or left open,[28] ranging from approximately three days[41] to three weeks.
[39] In rare circumstances, such as extensive cutaneous injury, self-renewal subpopulations in the bone marrow are induced to participate in the healing process, whereby they give rise to collagen-secreting cells that seem to play a role during wound repair.
[39] Moreover, it is thought that extensive injury to skin also promotes the early trafficking of a unique subclass of leukocytes (circulating fibrocytes) to the injured region, where they perform various functions related to wound healing.
[71] Repair or incomplete regeneration, refers to the physiologic adaptation of an organ after injury in an effort to re-establish continuity without regards to exact replacement of lost/damaged tissue.
[1] In addition to providing support for fibroblast and endothelial cell attachment, biodegradable scaffolds inhibit wound contraction, thereby allowing the healing process to proceed towards a more-regenerative/less-scarring pathway.
Historically, certain cultures consider scarification attractive;[81] however, this is generally not the case in the modern western society, in which many patients are turning to plastic surgery clinics with unrealistic expectations.
Scarless wound healing only occurs in mammalian foetal tissues[86] and complete regeneration is limited to lower vertebrates, such as salamanders, and invertebrates.
Clues as to how this might be achieved come from studies of wound healing in embryos, where repair is fast and efficient and results in essentially perfect regeneration of any lost tissue.
[88][89][90] After inflammation, restoration of normal tissue integrity and function is preserved by feedback interactions between diverse cell types mediated by adhesion molecules and secreted cytokines.
Disruption of normal feedback mechanisms in cancer threatens tissue integrity and enables a malignant tumor to escape the immune system.
[91][92] An example of the importance of the wound healing response within tumors is illustrated in work by Howard Chang and colleagues at Stanford University studying breast cancers.
The biologically active chemicals that play an important role in wound healing are modeled with Fickian diffusion to generate concentration profiles.
The balance equation for open systems when modeling wound healing incorporates mass growth due to cell migration and proliferation.
[99] Relationships like these can be incorporated into an agent-based models, where the sensitivity to single parameters such as initial collagen alignment, cytokine properties, and cell proliferation rates can be tested.
Primary intention can only be implemented when the wound is precise and there is minimal disruption to the local tissue and the epithelial basement membrane, e.g. surgical incisions.
[108] This includes a number of products under the trade names such as Epicel, Laserskin, Transcyte, Dermagraft, AlloDerm/Strattice, Biobrane, Integra, Apligraf, OrCel, GraftJacket and PermaDerm.