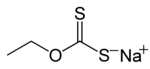
Xanthate
These organosulfur compounds are important in two areas: the production of cellophane and related polymers from cellulose and (in mining) for extraction of certain sulphide bearing ores.
[2] In chemical terminology, the alkali reacts with the alcohol to produce an alkoxide, which is the nucleophile that adds to the electrophilic carbon atom in CS2.
The methyl and ethyl xanthic acids are oils that are soluble in organic solvents.
[5] These compounds thermally decompose in the presence of base to the alcohol and carbon disulfide.
[6] Xanthic acids characteristically decompose: This reaction is the reverse of the method for the preparation of the xanthate salts.
They can be oxidized to dixanthogen disulfides: Acylation of xanthates gives alkyl xanthogen esters (ROC(S)SC(O)R') and related anhydrides.
They can be used to control radical polymerisation under the RAFT process, also termed MADIX (macromolecular design via interchange of xanthates).
Cellulose reacts with carbon disulfide (CS2) in presence of sodium hydroxide (NaOH) to produces sodium cellulose xanthate, which upon neutralization with sulfuric acid (H2SO4) gives viscose rayon or cellophane paper (Sellotape or Scotch Tape).