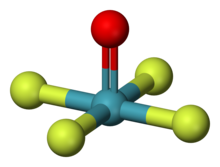
Xenon oxytetrafluoride
Xenon oxytetrafluoride (XeOF4) is an inorganic chemical compound.
It is an unstable colorless liquid[2][3] with a melting point of −46.2 °C (−51.2 °F; 227.0 K)[4] that can be synthesized by partial hydrolysis of XeF6, or the reaction of XeF6 with silica[3] or NaNO3:[5] A high-yield synthesis proceeds by the reaction of XeF6 with POF3 at −196 °C (−320.8 °F; 77.1 K).
[6] Like most xenon oxides, it is extremely reactive, and it hydrolyses in water to give hazardous and corrosive products, including hydrogen fluoride: In addition, some ozone and fluorine is formed.
XeOF4 reacts with H2O in the following steps: The XeO3 formed is a dangerous explosive, decomposing explosively to Xe and O2: In its liquid form, XeOF4 exhibits amphoteric behaviour, forming complexes with both strong Lewis bases like CsF and strong Lewis acids like SbF5.
[7] It forms a 1:1 adduct with XeF2, isostructural with XeF2·IF5,[8] as well as various heavy alkali metal fluorides.