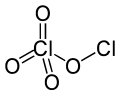
Chlorine perchlorate
Chlorine perchlorate is a chemical compound with the formula Cl2O4.
This chlorine oxide is an asymmetric oxide, with one chlorine atom in +1 oxidation state and the other +7, with proper formula ClOClO3.
It is produced by the photodimerization of chlorine dioxide (ClO2) at room temperature by 436 nm ultraviolet light:[2][3][4] Chlorine perchlorate can also be made by the following reaction at −45 °C.
Chlorine perchlorate is a pale greenish liquid.
It is less stable than ClO2 (chlorine dioxide)[citation needed] and decomposes at room temperature to give O2 (oxygen), Cl2 (chlorine) and Cl2O6 (dichlorine hexoxide): Chlorine perchlorate reacts with metal chlorides to form chlorine and the corresponding anhydrous perchlorate: