Zinc smelting
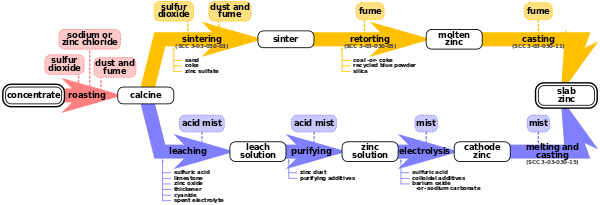
[1] In a multiple-hearth roaster, the concentrate drops through a series of 9 or more hearths stacked inside a brick-lined cylindrical column.
As the feed concentrate drops through the furnace, it is first dried by the hot gases passing through the hearths and then oxidized to produce calcine.
[1] In a fluidized-bed roaster, finely ground sulfide concentrates are suspended and oxidized in feedstock bed supported on an air column.
There is still cadmium, copper, arsenic, antimony, cobalt, germanium, nickel, and thallium in the leach product.
It uses zinc dust and steam to remove copper, cadmium, cobalt, and nickel, which would interfere with the electrolysis process.
Impurities can change the decomposition voltage enough to where the electrolysis cell produces largely hydrogen gas rather than zinc metal.
Every 24 to 48 hours, each cell is shut down, the zinc-coated cathodes are removed and rinsed, and the zinc is mechanically stripped from the aluminium plates.
The latter gives better purity and has higher production capacity per volume of electrolyte, but has the disadvantage of running hotter and being more corrosive to the vessel in which it is done.
In either of the electrolytic processes, each metric ton of zinc production expends about 3,900 kW⋅h (14 GJ) of electric power.
[6] Depending on the type of end-products produced, the zinc cathodes coming out of the electro-winning plant can undergo an additional transformation step in a foundry.
Finally, molten zinc may be transported to nearby conversion plants or third parties using specially-designed insulated containers.
The major downfall of any of the pyrometallurgical process is that it is only 98% pure; a standard composition is 1.3% lead, 0.2% cadmium, 0.03% iron, and 98.5% zinc.
The advantage of this system is that it is able to smelt a wide variety of zinc-bearing materials, including electric arc furnace dust.
A pair of graphite electrodes from the top and bottom of the furnace produce current flow through the mixture.
L. J. Derham proposed using a spray of molten lead droplets to rapidly cool and absorb the zinc vapour, despite the high concentration of carbon dioxide.
The process starts by charging solid sinter and heated coke into the top of the blast furnace.
A modified version of this process is still used at a Huludao plant in China (originally established by the Japanese in 1937), which produced 65,000 metric tons per year as of 1991[7] and increased capacity to at least 210,000 t/year by 2023.
There are three reasons to briquette the calcine: to ensure free downward movement of the charge; to permit heat transfer across a practical size cross-section; to allow adequate porosity for the passage of reduced zinc vapour to the top of the retort.
[7] This process was licensed to the Imperial Smelting Corporation (ISC), based in Avonmouth, England, which had a large vertical retort (VR) plant in production for many years.
Each retort only produced 40 kilograms (88 lb) so companies would put them together in banks and used one large gas burner to heat all of them.
[10] The Belgian process requires redistillation to remove impurities of lead, cadmium, iron, copper, and arsenic.
Smelting is thought to have been done in sealed cylindrical clay retorts which were packed with a mixture of roasted ore, dolomite, and an organic material, perhaps cow dung, and then placed vertically in a furnace and heated to around 1100 °C.
[15] In Champion's process, zinc ore (in this case, the carbonate, ZnCO3) was sealed in large reduction pots with charcoal and heated in a furnace.
The zinc vapor then descended through an iron condensing pipe until reaching a water-filled vessel at the bottom.
In the Carinthian process, used in works established in 1798 by Bergrath Dillinger, a wood-fueled furnace heated a large number of small vertical retorts,[20] and zinc vapor then dropped through a ceramic pipe into a common condensation chamber below.
[22] The merged "Belgo-Silesian" horizontal retort process was widely adopted in Europe by the third quarter of the 19th century, and later in the United States.
Gaseous zinc was drawn off from the top of the column and, after a 20-hour journey through the retort, spent briquettes were removed from the bottom.
[26] To condense the gaseous zinc, the company first used a simple brick chamber with carborundum baffles, but efficiency was poor.
[26][28] An efficient condenser was devised for this process from 1931–1936; it consisted of a bath of liquid zinc which the exhaust gases were drawn through by suction.
[27] The blast-furnace process was developed starting in 1943 at Avonmouth, England by the Imperial Smelting Corporation,[29] which became part of Rio Tinto Zinc in 1968.