Zirconium(IV) iodide
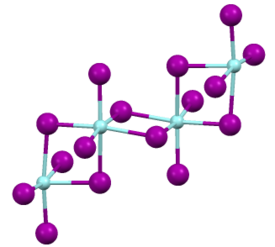
Like most binary metal halides, zirconium(IV) iodide adopts a polymeric structure.
As characterized by X-ray crystallography, the compound consists of octahedral Zr(IV) centers interconnected by four doubly bridging iodide ligands.
The Zr-I distances of 2.692 (terminal) and 3.030 Å[2][3] This compound can be prepared by heating zirconium metal and an excess of iodine.
[4] Pyrolysis of zirconium(IV) iodide gas by contact with a hot wire was the first industrial process for the commercial production of pure ductile metallic zirconium.
This crystal bar process was developed by Anton Eduard van Arkel and Jan Hendrik de Boer in 1925.