Zirconium(IV) chloride
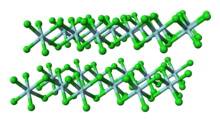
Unlike molecular TiCl4, solid ZrCl4 adopts a polymeric structure wherein each Zr is octahedrally coordinated.
This polymer degrades readily upon treatment with Lewis bases, which cleave the Zr-Cl-Zr linkages.
For their conversion to bulk metal, these refractory oxides are first converted to the tetrachloride, which can be distilled at high temperatures.
This reaction is rapid and virtually irreversible, consistent with the high oxophilicity of zirconium(IV).
[9] Sodium cyclopentadienide (NaC5H5) reacts with ZrCl4(THF)2 to give zirconocene dichloride, ZrCl2(C5H5)2, a versatile organozirconium complex.