1,1,1-Trichloroethane
Stabilizers comprise up to 8% of the formulation, including acid scavengers (epoxides, amines) and complexants.
It is generally considered non-polar, but owing to the good polarizability of the chlorine atoms, it is a superior solvent for organic compounds that do not dissolve well in hydrocarbons such as hexane.
Prior to the Montreal Protocol, it was widely used for cleaning metal parts and circuit boards, as a photoresist solvent in the electronics industry, as an aerosol propellant, as a cutting fluid additive, and as a solvent for inks, paints, adhesives, and other coatings.
[8] In the 1880s, it was found to be a safe and strong substitute for chloroform[9] but its production was too expensive and difficult for the era.
At the time, the narcotic effects of chloral hydrate were owed to a hypothetical metabolic pathway to chloroform in "alkaline blood".
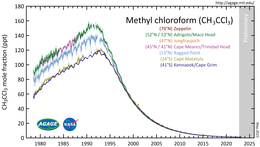
[17] 1,1,1-Trichloroethane is a fairly potent greenhouse gas with a 100-year global warming potential of 169 relative to carbon dioxide.
[18] This is nonetheless less than a tenth that of carbon tetrachloride — which it replaced as a solvent — due to its relatively short atmospheric lifetime of about 5 years.
[19] The Montreal Protocol targeted 1,1,1-trichloroethane as a compound responsible for ozone depletion and banned its use beginning in 1996.