1,4-Butane sultone
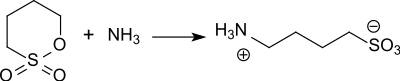
In such, the sulfobutyl group is present as neutral sodium salt and considerably increases the water solubility of the derivatives.
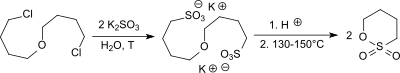
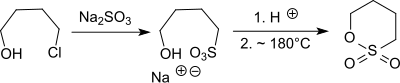
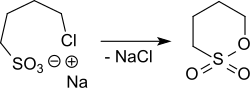
A lab scale synthesis of 1,4-butanesultone starts from 4,4'-dichlorodibutyl ether (accessible from tetrahydrofuran treated with phosphorus oxychloride and concentrated sulfuric acid),[3][4] which reacts with sodium sulfite forming the corresponding 4,4'-butanedisulfonic disodium salt.
[9] The free-radical initiated sulfochlorination of 1-chlorobutane leads to a mixture of positionally isomeric sulfochlorides and chlorination products and is therefore not suitable for the direct preparation of 1,4-butanesultone.
[11] [3] Compared to the homologous γ-sultone 1,3-propanesultone, 1,4-butanesultone is significantly less reactive as alkylating agent, but classified as mutagenic and carcinogenic.
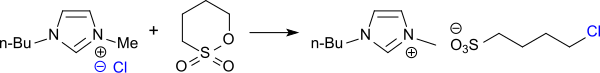
[16] Sulfobutylation of cyanine dyes leads to readily water-soluble compounds which react with proteins like antibodies and can be used as pH-sensitive fluorescence markers.
[19] Already in 1949 the reaction of 1,4-butanesultone with the water-insoluble polysaccharide cellulose in sodium hydroxide solution was reported, which leads to a water-soluble product.