Water softening
Soft water also extends the lifetime of plumbing by reducing or eliminating scale build-up in pipes and fittings.
The presence of certain metal ions like calcium and magnesium, principally as bicarbonates, chlorides, and sulfates, in water causes a variety of problems.
[1] Hard water leads to the buildup of limescale, which can foul plumbing, and promote galvanic corrosion.
The surface of human skin has a light charge that the soap tends to bind with, requiring more effort and a greater volume of water to remove.
[4][5] The most common means for removing water hardness rely on ion-exchange resin or reverse osmosis.
Other approaches include precipitation methods, such as fluidized bed pellet softening,[6] and sequestration by the addition of chelating agents.
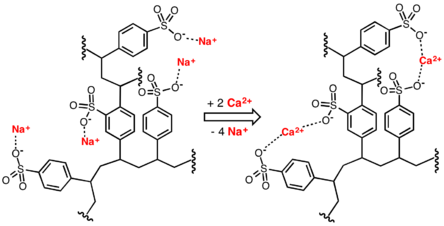
[7] As described by NSF/ANSI Standard 44,[8] ion-exchange devices reduce the hardness by replacing magnesium and calcium (Mg2+ and Ca2+) with sodium or potassium ions (Na+ and K+)."
The waste waters eluted from the ion-exchange column containing the unwanted calcium and magnesium salts are typically discharged to the sewage system.
[13] Chelators are used in chemical analysis, as water softeners, and are ingredients in many commercial products such as shampoos and food preservatives.
Due to environmental and aquatic toxicity concerns regarding widespread use of EDTA in household and personal care products, alternatives such as sodium phytate/phytic acid, tetrasodium glutamate diacetate and trisodium ethylenediamine disuccinate are finding more prevalent usage.
In this method, water is treated with a calculated amount of washing soda (Na2CO3), which converts the chlorides and sulphates of calcium and magnesium into their respective carbonates, which get precipitated.
The process is often used in conjunction with reverse osmosis filtration, as nanofiltration on its own is not as effective and more expensive than chemical water treatment methods.
The dissolved minerals become insoluble solid particles in suspension, passing through the system without binding to plumbing surfaces.
Testing at the University of Arizona found TAC to be the most effective at reducing scale formation, followed closely by ion exchange (see chart above).
The advantages of TAC tanks include simplicity, low maintenance, lack of toxic effluent (like chlorine), and the availability of calcium as a nutrient in drinking water.
For people with impaired kidney function, however, elevated potassium levels, or hyperkalemia, can lead to complications such as cardiac arrhythmia.
[citation needed] High levels of water hardness in the home may also be linked to the development of atopic dermatitis (eczema) early in life,[25] although the actual relationship is correlational at the present and further research is indicated to establish a causal one.