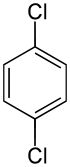
1,4-Dichlorobenzene
[3] p-DCB is produced by chlorination of benzene using ferric chloride as a catalyst: The chief impurity is the 1,2 isomer.
Its usefulness for these applications arises from p-DCB's low solubility in water and its relatively high volatility: it sublimes readily near room temperature.
The United States Department of Health and Human Services (DHHS) and the International Agency for Research on Cancer (IARC) have determined that p-DCB may reasonably be anticipated to be a carcinogen.
[8] The United States Environmental Protection Agency (EPA) has set a target maximum contaminant level of 75 micrograms of p-DCB per liter of drinking water (75 μg/L),[9] but publishes no information on the cancer risk.
[14] Due to its carcinogenic nature, use of paradichlorobenzene in the European Union is forbidden as an air freshener (since 2005) and in mothballs (since 2008).