Carbon
Graphite is soft enough to form a streak on paper (hence its name, from the Greek verb "γράφειν" which means "to write"), while diamond is the hardest naturally occurring material known.
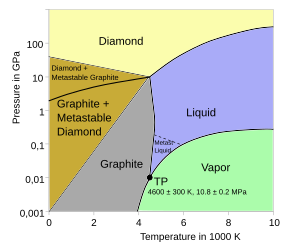
All carbon allotropes are solids under normal conditions, with graphite being the most thermodynamically stable form at standard temperature and pressure.
[22][23] Graphite is much more reactive than diamond at standard conditions, despite being more thermodynamically stable, as its delocalised pi system is much more vulnerable to attack.
The electronegativity of carbon is 2.5, significantly higher than the heavier group-14 elements (1.8–1.9), but close to most of the nearby nonmetals, as well as some of the second- and third-row transition metals.
[43] The amorphous form is an assortment of carbon atoms in a non-crystalline, irregular, glassy state, not held in a crystalline macrostructure.
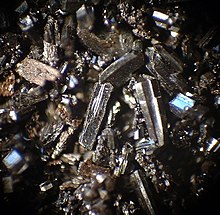
At normal pressures, carbon takes the form of graphite, in which each atom is bonded trigonally to three others in a plane composed of fused hexagonal rings, just like those in aromatic hydrocarbons.
Because of the delocalization of one of the outer electrons of each atom to form a π-cloud, graphite conducts electricity, but only in the plane of each covalently bonded sheet.
Diamond has the same cubic structure as silicon and germanium, and because of the strength of the carbon-carbon bonds, it is the hardest naturally occurring substance measured by resistance to scratching.
The properties of fullerenes (split into buckyballs, buckytubes, and nanobuds) have not yet been fully analyzed and represent an intense area of research in nanomaterials.
[31] Carbon nanotubes (buckytubes) are structurally similar to buckyballs, except that each atom is bonded trigonally in a curved sheet that forms a hollow cylinder.
[32][33] Nanobuds were first reported in 2007 and are hybrid buckytube/buckyball materials (buckyballs are covalently bonded to the outer wall of a nanotube) that combine the properties of both in a single structure.
[50] In 2015, a team at the North Carolina State University announced the development of another allotrope they have dubbed Q-carbon, created by a high-energy low-duration laser pulse on amorphous carbon dust.
[65] As for individual carbon allotropes, graphite is found in large quantities in the United States (mostly in New York and Texas), Russia, Mexico, Greenland, and India.
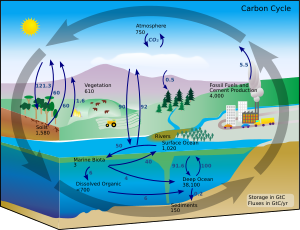
[72] It is found in trace amounts on Earth of 1 part per trillion (0.0000000001%) or more, mostly confined to the atmosphere and superficial deposits, particularly of peat and other organic materials.
[74][75] There are 15 known isotopes of carbon and the shortest-lived of these is 8C which decays through proton emission and has a half-life of 3.5×10−21 s.[15] The exotic 19C exhibits a nuclear halo, which means its radius is appreciably larger than would be expected if the nucleus were a sphere of constant density.
[77] The triple-alpha process happens in conditions of temperatures over 100 megakelvins and helium concentration that the rapid expansion and cooling of the early universe prohibited, and therefore no significant carbon was created during the Big Bang.
Common heteroatoms that appear in organic compounds include oxygen, nitrogen, sulfur, phosphorus, and the nonradioactive halogens, as well as the metals lithium and magnesium.
When united with hydrogen, it forms various hydrocarbons that are important to industry as refrigerants, lubricants, solvents, as chemical feedstock for the manufacture of plastics and petrochemicals, and as fossil fuels.
When combined with oxygen and hydrogen, carbon can form many groups of important biological compounds including sugars, lignans, chitins, alcohols, fats, aromatic esters, carotenoids and terpenes.
With the addition of phosphorus to these other elements, it forms DNA and RNA, the chemical-code carriers of life, and adenosine triphosphate (ATP), the most important energy-transfer molecule in all living cells.
[104] In nature, the iron-molybdenum cofactor (FeMoco) responsible for microbial nitrogen fixation likewise has an octahedral carbon center (formally a carbide, C(-IV)) bonded to six iron atoms.
Diamonds were known probably as early as 2500 BCE in China, while carbon in the form of charcoal was made by the same chemistry as it is today, by heating wood in a pyramid covered with clay to exclude air.
[112] In 1786, the French scientists Claude Louis Berthollet, Gaspard Monge and C. A. Vandermonde confirmed that graphite was mostly carbon by oxidizing it in oxygen in much the same way Lavoisier had done with diamond.
[38] Commercially viable natural deposits of graphite occur in many parts of the world, but the most important sources economically are in China, India, Brazil, and North Korea.
[116] Graphite deposits are of metamorphic origin, found in association with quartz, mica, and feldspars in schists, gneisses, and metamorphosed sandstones and limestone as lenses or veins, sometimes of a metre or more in thickness.
The ore is crushed, during which care has to be taken in order to prevent larger diamonds from being destroyed in this process and subsequently the particles are sorted by density.
[127] In 2004, a startling discovery of a microscopic diamond in the United States[128] led to the January 2008 bulk-sampling of kimberlite pipes in a remote part of Montana.
The major economic use of carbon other than food and wood is in the form of hydrocarbons, most notably the fossil fuel methane gas and crude oil (petroleum).
It is also used as a lubricant and a pigment, as a moulding material in glass manufacture, in electrodes for dry batteries and in electroplating and electroforming, in brushes for electric motors, and as a neutron moderator in nuclear reactors.
Carbon compounds make up most of the materials used in clothing, such as natural and synthetic textiles and leather, and almost all of the interior surfaces in the built environment other than glass, stone, drywall, and metal.