Volatility (chemistry)
[1] Differences in volatility can be observed by comparing how fast substances within a group evaporate (or sublimate in the case of solids) when exposed to the atmosphere.
A substance enclosed in a sealed vessel initially at vacuum (no air inside) will quickly fill any empty space with vapor.
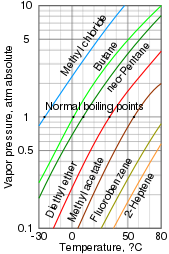
In general, volatility tends to decrease with increasing molecular mass because larger molecules can participate in more intermolecular bonding,[5] although other factors such as structure and polarity play a significant role.
The effect of molecular mass can be partially isolated by comparing chemicals of similar structure (i.e. esters, alkanes, etc.).
For instance, linear alkanes exhibit decreasing volatility as the number of carbons in the chain increases.
[6] The process of petroleum refinement utilizes a technique known as fractional distillation, which allows several chemicals of varying volatility to be separated in a single step.
The crude oil flows into a distillation tower and is heated up, which allows the more volatile components such as butane and kerosene to vaporize.
These vapors move up the tower and eventually come in contact with cold surfaces, which causes them to condense and be collected.
[1] The difference in volatility between water and ethanol has long been used to produce concentrated alcoholic beverages (many of these are referred to as "liquors").