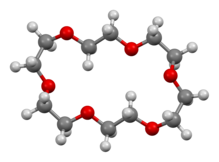
18-Crown-6
[3] Rigorously dry material can be made by dissolving the compound in THF followed by the addition of NaK to give [K(18-crown-6)]Na, an alkalide salt.
[4] Crystallographic analysis reveals a relatively flat molecule but one where the oxygen centres are not oriented in the idealized 6-fold symmetric geometry usually shown.
For example, interaction of 18-crown-6 with HCl gas in toluene with a little moisture gives an ionic liquid layer with the composition [H3O·18-crown-6]+[HCl2]−·3.8C6H5Me, from which the solid [H3O·18-crown-6]+[HCl2]− can be isolated on standing.
For example, using 18-crown-6, potassium acetate is a more powerful nucleophile in organic solvents:[1] The first electride salt to be examined with X-ray crystallography, [Cs(18-crown-6)2]+·e−, was synthesized in 1983.
This highly air- and moisture-sensitive solid has a sandwich molecular structure, where the electron is trapped within nearly spherical lattice cavities.