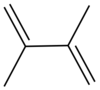
Dimethylbutadiene
It is colorless liquid which served an important role in the early history of synthetic rubber.
Dimethylbutadiene is readily prepared by an acid catalyzed dehydration reaction of pinacol:[3] The current industrial route involves dimerization of propene followed by dehydrogenation.
[4] In 1909, Fritz Hofmann and a team working at Bayer succeeded in polymerizing dimethylbutadiene.
[4] Dimethylbutadiene readily undergoes Diels-Alder reactions and reacts faster than 1,3-butadiene.
Its effectiveness in this reaction is attributed to the stabilization of the cis-conformation owing to the influence of the methyl groups on the C2 and C3 positions.