2,5-Diketopiperazine
These range from the hepatoxic brevianamide F (cyclo(L-Trp-L-Pro)) to the annulated tremorogenic verruculogen and the spiro-annulated spirotryprostatin B which represent a promising class of antimitotic arrest agents, to the structurally complex (+)-stephacidin A, a bridged 2,5-diketopiperazine that possess a unique bicyclo[2.2.2]diazaoctane core ring system and is active against the human colon HCT-116 cell line.
The unsaturated derivatives are illustrated by phenylahistin the anti-cancer microtubule binding agent, and the mycotoxin roquefortine C found in blue cheeses.
They have been detected in stewed beef, beer, bread, Awamori spirits, cocoa, chicken essence, roasted coffee, Comte cheese, dried squid, aged saki and yeast extract.
In food systems, 2,5-diketopiperazines have been shown to be important sensory compounds contributing to the taste of the final products and being perceived as astringent, salty, grainy, metallic or bitter.
[3] It has also been isolated from a variety of marine microorganisms and has been identified as an active LasI quorum-sensing signal molecule important for the plant growth promotion by Pseudomonas aeruginosa.
It also occurs in humans[6] as a metabolite from the thyrotropin-releasing hormone (TRH) and exhibits a wide variety of central nervous system, endocrine, electrophysiological, and cardiovascular effects.
As a consequence of their predominant biosynthetic origin from L-α-amino acids most naturally occurring 2,5-DKPs are cis configured as the cyclo(L-Xaa-L-Yaa) isomers.
The composition of the cis and trans isomers in the equilibrium state varies widely depending on the bulk of the side chains, if a ring (e.g. proline) is present, or if the nitrogen atoms are alkylated .
In general, they arise by the action of a tRNA-dependent cyclodipeptide synthases, a type of enzyme responsible for creating a cyclic amide linkage between two peptides.
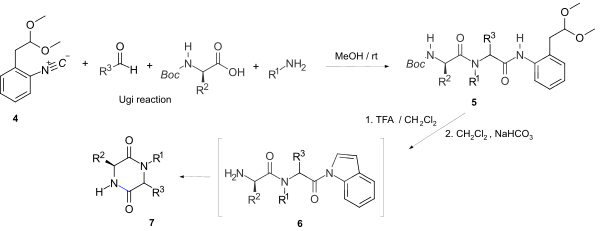
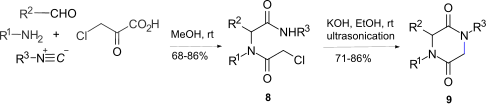
[9] The Ugi reaction using an isonitrile, amino acid, aldehyde and amine, can produce a dipeptide in equally high yield and optical purity, to that formed by standard peptide couplings.
For example, the direct 2,5-DKP ring formation via such an activated leaving group using the stable, easily accessible and versatile convertible isonitrile 1-isocyano-2-(2,2-dimethoxyethyl)-benzene 4 gave a one-pot synthesis of N-substituted 2,5-diketopiperazine's 7.
For example, the 2,5-DKP cyclo(Phe-Pro) has been shown to play a role in the regulation of gene expression in multiple different species of bacteria including V. fishceri, V. cholera, Lactobacillus reuteri, Staphylococcus aureus, among others.
These characteristics not only enable them to bind with high affinity to a large variety of receptors, showing a broad range of biological activities, but also allow the development of the drug-like physicochemical properties required for the multiobjective optimization process of transforming a lead to a drug product.
These chemicals can be used to imitate quorum sensing signals to regulate gene expression of pathogenic bacteria and help fight against bacterial infection.