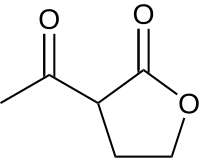
2-Acetylbutyrolactone
2-Acetylbutyrolactone (ABL) is a derivative of γ-butyrolactone that is used as a precursor in organic synthesis, and it is used to identify primary amines through chemical fluorescence.
[5] 2-Acetylbutyrolactone can also be prepared by reacting ethylene oxide with ethyl acetoacetate in alkaline conditions.
[3][6] The carbonyl group readily reacts with amines to form Schiff bases.
It is for this reason that 2-acetylbutyrolactone is frequently used to confirm the creation of amines during organic synthesis.
[3] 2-Acetylbutyrolactone can also undergo a Japp–Klingemann reaction to form fluorescent molecules with arylamines.