2-Ethyl-2-oxazoline
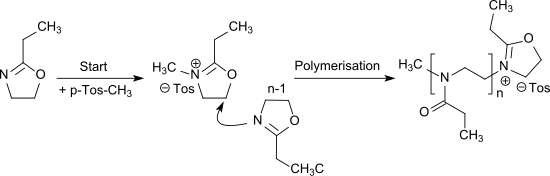
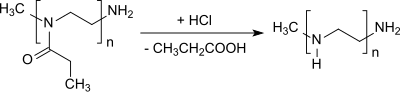
2-Ethyl-2-oxazoline (EtOx) is an oxazoline which is used particularly as a monomer for the cationic ring-opening polymerization to poly(2-alkyloxazoline)s.[2] This type of polymers are under investigation as readily water-soluble and biocompatible materials for biomedical applications.
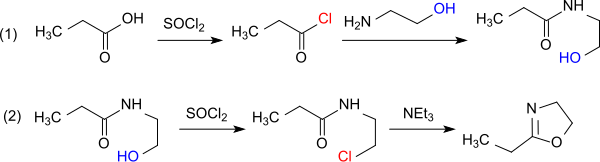
[4] Less drastic reaction conditions require the dehydration of the N-(2-hydroxyethyl)propionamide in vacuo in the presence of iron(III)chloride, which delivers the product in 90% yield.
[6] An economic one-pot reaction is heating the salt of propionic acid with ethanolamine at 200 °C in vacuo in the presence of zinc chloride yielding 82% 2-ethyl-2-oxazoline.
Water must be excluded due to the tendency of oxazolines towards ring-opening by chloride ions during protonation of the imine nitrogen.
[10] 2-Ethyl-2-oxazoline is a readily water-soluble, colorless liquid which is also soluble in a variety of organic solvents and possesses an amine-like smell.