Zinc chloride
Zinc chloride is an inorganic chemical compound with the formula ZnCl2·nH2O, with n ranging from 0 to 4.5, forming hydrates.
Zinc chloride, anhydrous and its hydrates, are colorless or white crystalline solids, and are highly soluble in water.
[5] Zinc chloride has long been known but currently practiced industrial applications all evolved in the latter half of 20th century.
[13] Molten ZnCl2 has a high viscosity at its melting point and a comparatively low electrical conductivity, which increases markedly with temperature.
[16] Neutron scattering study indicated the presence of tetrahedral ZnCl4 centers, which requires aggregation of ZnCl2 monomers as well.
[18] The 1.33-hydrate, previously thought to be the hemitrihydrate, consists of trans-Zn(H2O)4Cl2 centers with the chlorine atoms connected to repeating ZnCl4 chains.
The trihydrate consists of distinct hexaaquozinc(II) cations and tetrachlorozincate anions; formulated [Zn(H2O)6][ZnCl4].
Finally, the heminonahydrate, structurally formulated [Zn(H2O)6][ZnCl4]·3H2O also consists of distinct hexaaquozinc(II) cations and tetrachlorozincate anions like the trihydrate but has three extra water molecules.
[20] Commercial samples of zinc chloride typically contain water and products from hydrolysis as impurities.
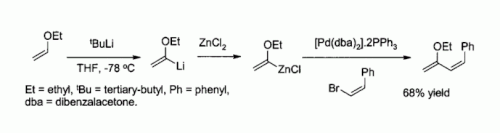
Being soluble in ethers and lacking acidic protons, this complex is used in the synthesis of organozinc compounds.
[30] The distinctive ability of aqueous solutions of ZnCl2 to dissolve cellulose is attributed to the formation of zinc-cellulose complexes, illustrating the stability of its adducts.
When solutions of zinc chloride are treated with ammonia, diverse ammine complexes are produced.
[42] In aqueous solution ZnCl2, as well as other halides (bromide, iodide), behave interchangeably for the preparation of other zinc compounds.
[18] Although Zn2+2 is unusual, mercury, a heavy congener of zinc, forms a wide variety of Hg2+2 salts.
[46] The ability of zinc chloride to dissolve metal oxides (MO)[47] is relevant to the utility of ZnCl2 as a flux for soldering.
A dramatic example is the conversion of methanol into hexamethylbenzene using zinc chloride as the solvent and catalyst:[48] This kind of reactivity has been investigated for the valorization of C1 precursors.
[49] Examples of zinc chloride as a Lewis acid include the Fischer indole synthesis:[50] Related Lewis-acid behavior is illustrated by a traditional preparation of the dye fluorescein from phthalic anhydride and resorcinol, which involves a Friedel-Crafts acylation.
[52][53] Zinc chloride also activates benzylic and allylic halides towards substitution by weak nucleophiles such as alkenes:[54] In similar fashion, ZnCl2 promotes selective Na[BH3(CN)] reduction of tertiary, allylic or benzylic halides to the corresponding hydrocarbons.
The prominence of this reaction was highlighted by the award of the 2010 Nobel Prize in Chemistry to Ei-ichi Negishi.
[58] Zinc chloride is used as a catalyst or reagent in diverse reactions conducted on an industrial scale.
The action of zinc chloride/ammonium chloride fluxes, for example, in the hot-dip galvanizing process produces H2 gas and ammonia fumes.
[5] Anhydrous zinc chloride is however an aggressive Lewis acid as it can burn skin and other tissues.