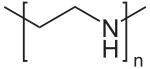
Polyethylenimine
Polyethylenimine (PEI) or polyaziridine is a polymer with repeating units composed of the amine group and two carbon aliphatic CH2CH2 spacers.
[10] Owing to its ability to modify the surface of cellulose fibres, PEI is employed as a wet-strength agent in the paper-making process.
First use of PEI polymer in CO2 capture was devoted to improve the CO2 removal in space craft applications, impregnated over a polymeric matrix.
[20] After that, the support was changed to MCM-41, an hexagonal mesostructured silica, and large amounts of PEI were retained in the so-called "molecular basket".
The authors claim that, in this case, a synergic effect takes place due to the high PEI dispersion inside the pore structure of the material.
[25] However, for an appropriate performance under real conditions in post-combustion capture (mild temperatures between 45-75 °C and the presence of moisture) it is necessary to use thermally and hydrothermally stable silica materials, such as SBA-15,[26] which also presents an hexagonal mesostructure.
[27] A detailed comparison among PEI and other amino-containing molecules showed an excellent performance of PEI-containing samples with cycles.
Also, only a slight decrease was registered in their CO2 uptake when increasing the temperature from 25 to 100 °C, demonstrating a high contribution of chemisorption to the adsorption capacity of these solids.
Amino groups incorporated by both paths have shown synergic effects, achieving high CO2 uptakes up to 235 mg CO2/g (5.34 mmol CO2/g).
Poly(ethylenimine) and poly(ethylenimine) ethoxylated (PEIE) have been shown as effective low-work function modifiers for organic electronics by Zhou and Kippelen et al.[34] It could universally reduce the work function of metals, metal oxides, conducting polymers and graphene, and so on.
Polyethylenimine (PEI), a cationic polymer, has been widely studied and shown great promise as an efficient gene delivery vehicle.