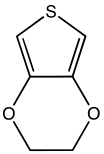
3,4-Ethylenedioxythiophene
The molecule consists of thiophene, substituted at the 3 and 4 positions with an ethylene glycolyl unit.
EDOT is often prepared from C4 precursors such as butanediol and butadiene via routes that produce the thiophene and dioxane rings in separate steps.
Representative is the reaction of 2,3-butanedione, trimethyl orthoformate, and ethylene glycol to form the dioxane.
[6] EDOT is converted into the conducting polymer PEDOT by oxidation.
The idealized conversion using peroxydisulfate is shown For commercial purposes, the polymerization is conducted in the presence of polystyrenesulfonate.