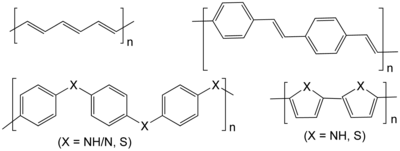
Conductive polymer
They can offer high electrical conductivity but do not show similar mechanical properties to other commercially available polymers.
[4] Polyaniline was first described in the mid-19th century by Henry Letheby, who investigated the electrochemical and chemical oxidation products of aniline in acidic media.
[6] In the 1950s, researchers reported that polycyclic aromatic compounds formed semi-conducting charge-transfer complex salts with halogens.
[3] In 1954, researchers at Bell Labs and elsewhere reported organic charge transfer complexes with resistivities as low as 8 Ω.cm.
While organic conductors were previously intermittently discussed, the field was particularly energized by the prediction of superconductivity[10] following the discovery of BCS theory.
[11] [7] With the notable exception of charge transfer complexes (some of which are even superconductors), organic molecules were previously considered insulators or at best weakly conducting semiconductors.
"[15] Polyacetylene itself did not find practical applications, but drew the attention of scientists and encouraged the rapid growth of the field.
[5] Since the late 1980s, organic light-emitting diodes (OLEDs) have emerged as an important application of conducting polymers.
Poly(p-phenylene vinylene) (PPV) and its soluble derivatives have emerged as the prototypical electroluminescent semiconducting polymers.
Non-doping increases in conductivity can also be accomplished in a field effect transistor (organic FET or OFET) and by irradiation.
Some materials also exhibit negative differential resistance and voltage-controlled "switching" analogous to that seen in inorganic amorphous semiconductors.
Conductive polymers show promise in antistatic materials[3] and they have been incorporated into commercial displays and batteries.
Literature suggests they are also promising in organic solar cells, printed electronic circuits, organic light-emitting diodes, actuators, electrochromism, supercapacitors, chemical sensors, chemical sensor arrays, and biosensors,[28] flexible transparent displays, electromagnetic shielding and possibly replacement for the popular transparent conductor indium tin oxide.
Conducting polymers are rapidly gaining attraction in new applications with increasingly processable materials with better electrical and physical properties and lower costs.
Research reports showed that nanostructured conducting polymers in the form of nanofibers and nanosponges exhibit significantly improved capacitance values as compared to their non-nanostructured counterparts.
While PEDOT (poly(3,4-ethylenedioxythiophene)) is mainly used in antistatic applications and as a transparent conductive layer in form of PEDOT:PSS dispersions (PSS=polystyrene sulfonic acid), polyaniline is widely used for printed circuit board manufacturing – in the final finish, for protecting copper from corrosion and preventing its solderability.
[4] Moreover, polyindole is also starting to gain attention for various applications due to its high redox activity,[31] thermal stability,[30] and slow degradation properties than competitors polyaniline and polypyrrole.
In some cases, similar light emission is observed when a voltage is applied to a thin layer of a conductive organic polymer film.
Such materials are salt-like (polymer salt), which makes them less soluble in organic solvents and water and hence harder to process.
Experimental and theoretical thermodynamical evidence suggests that conductive polymers may even be completely and principally insoluble so that they can only be processed by dispersion.
[34] Recently (as of 2020), researchers from IMDEA Nanoscience Institute reported experimental demonstration of the rational engineering of 1D polymers that are located near the quantum phase transition from the topologically trivial to non-trivial class, thus featuring a narrow bandgap.