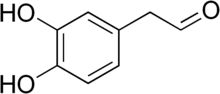
3,4-Dihydroxyphenylacetaldehyde
[2][4][3] According to the catecholaldehyde hypothesis, DOPAL plays a role in aging-related dopaminergic neurodegeneration and in the pathogenesis of Parkinson's disease.
[2][4][3] DOPAL is a metabolite of dopamine formed by monoamine oxidase (MAO).
[2] In differentiated neuronal cells of the PC12 line, physiological concentrations of DOPAL in isolated mitochondria were highly potent in inducing a pathway associated with programmed cell death (or apoptosis), permeability transition.
This suggests the cytotoxity of DOPAL and its role in the progression of Parkinson's disease, which has long been associated with mitochondrial abnormalities and neurotoxicity by way of dopaminergic compounds, while reducing the emphasis on other dopamine derivatives and metabolites.
[6] Aldehyde dehydrogenase inhibitors (ALDH inhibitors), which prevent the catabolism of DOPAL and thereby increase DOPAL levels, can produce dopaminergic neurotoxicity or augment dopaminergic neurodegeneration.