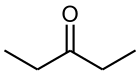
3-Pentanone
3-Pentanone is produced by ketonic decarboxylation of propanoic acid using metal oxide catalysts: in the laboratory, the reaction can be conducted in a tube furnace.
[4] When the reaction is catalyzed by dicobalt octacarbonyl, water can be used as a source of hydrogen.
A proposed intermediate is the ethylene-propionyl species [CH3C(O)Co(CO)3(ethylene)] which undergoes a migratory insertion to form [CH3COCH2CH2Co(CO)3].
Although considered stable, 3-pentanone is extremely flammable if exposed to flame, sparks, or another source of heat.
For safety, it should be stored in a flammable materials cabinet away from heat or sources of ignition, preferably in a cool, well-ventilated area.