Water–gas shift reaction
The WGSR is a highly valuable industrial reaction that is used in the manufacture of ammonia, hydrocarbons, methanol, and hydrogen.
Its most important application is in conjunction with the conversion of carbon monoxide from steam reforming of methane or other hydrocarbons in the production of hydrogen.
[2] Unfortunately, current commercial catalysts that are used in industrial water gas shift processes are not compatible with fuel cell applications.
Since the WGSR is slow at lower temperatures where equilibrium favors hydrogen production, WGS reactors require large amounts of catalysts, which increases their cost and size beyond practical application.
[2] The commercial LTS catalyst used in large scale industrial plants is also pyrophoric in its inactive state and therefore presents safety concerns for consumer applications.
The WGS reaction is used in combination with the solid adsorption of CO2 in the sorption enhanced water gas shift (SEWGS) in order to produce a high pressure hydrogen stream from syngas.
[6] The initial HTS takes advantage of the high reaction rates, but results in incomplete conversion of carbon monoxide.
The typical composition of commercial HTS catalyst has been reported as 74.2% Fe2O3, 10.0% Cr2O3, 0.2% MgO (remaining percentage attributed to volatile components).
[7] The search for high performance HT WGS catalysts remains an intensive topic of research in fields of chemistry and materials science.
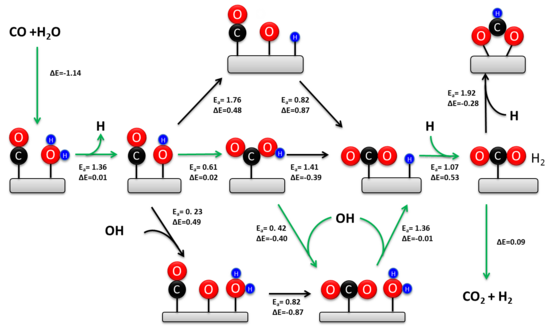
The redox mechanism is generally regarded as kinetically relevant during the high-temperature WGSR (> 350 °C) over the industrial iron-chromia catalyst.
Recent experimental studies confirm that the associative carboxyl mechanism is the predominant low temperature pathway on metal-oxide-supported transition metal catalysts.
[15][13] However, the carboxyl pathway accounts for about 90% of the total rate owing to the thermodynamic stability of adsorbed formate on the oxide support.
The formate intermediate can be eliminated during the WGSR by using oxide-supported atomically dispersed transition metal catalysts, further confirming the kinetic dominance of the carboxyl pathway.
A water molecule undergoes dissociative adsorption at the newly formed O-vacancy to yield two hydroxyls.
The mechanism entails nucleophilic attack of water or hydroxide on a M-CO center, generating a metallacarboxylic acid.