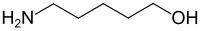
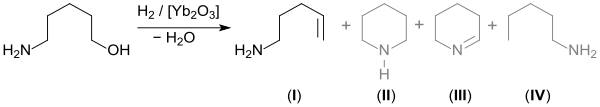5-Amino-1-pentanol
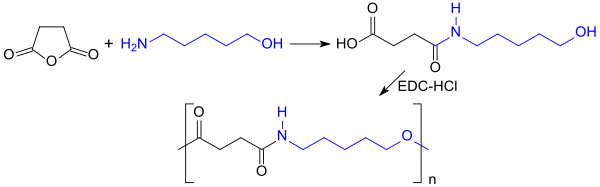
As a derivative of the platform chemical furfural (that is easily accessible from pentoses), 5-amino-1-pentanol may become increasingly important in the future as a building block for biodegradable polyesteramides and as a starting material for valerolactam — the monomer for polyamides.
The complete hydrogenation of furfural (furan-2-aldehyde) yields tetrahydrofurfuryl alcohol (2-hydroxymethyltetrahydrofuran), which undergoes ring expansion upon dehydration to give dihydropyran.
Similarly, the hemiacetal 2-hydroxytetrahydropyran[3] that is formed from dihydropyran with hydrochloric acid can be converted to 5-amino-1-pentanol by reductive amidation with ammonia and hydrogen upon water elimination.
[4] 5-Amino-1-pentanol forms white crystalline clumps at solidification temperatures around 35 °C, which dissolve in water, ethanol, and acetone.
In a dehydrogenation, catalyzed by rhodium and ruthenium complexes, valerolactam, the δ-lactam of 5-aminopentanoic acid, is formed from 5-amino-1-pentanol in high (94%) yield.