1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide
It is generally used as a carboxyl activating agent for the coupling of primary amines to yield amide bonds.
Common uses for this carbodiimide include peptide synthesis, protein crosslinking to nucleic acids, but also in the preparation of immunoconjugates.
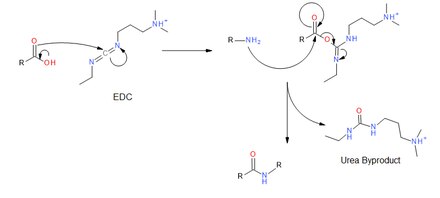
It may be prepared by coupling ethyl isocyanate to N,N-dimethylpropane-1,3-diamine to give a urea, followed by a dehydration reaction mediated by TsCl and TEA:[4] EDC couples primary amines, and other nucleophiles,[5] to carboxylic acids by creating an activated ester leaving group.
The primary amine then attacks the carbonyl carbon of the acid which forms a tetrahedral intermediate before collapsing and discharging the urea byproduct.
[8] Thermal hazard analysis by differential scanning calorimetry (DSC) shows EDC poses minimal explosion risks.