Bioenergetic systems
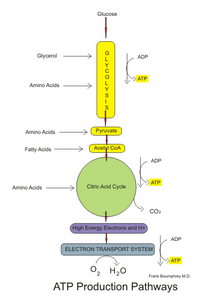
Those processes convert energy into adenosine triphosphate (ATP), which is the form suitable for muscular activity.
During exercise, the supply and demand of oxygen available to muscle cells is affected by duration and intensity and by the individual's cardio respiratory fitness level.
[2][3] Three systems can be selectively recruited, depending on the amount of oxygen available, as part of the cellular respiration process to generate ATP for the muscles.
Other forms of chemical energy, such as those available from oxygen and food, must be transformed into ATP before they can be utilized by the muscle cells.
The building blocks of ATP synthesis are the by-products of its breakdown; adenosine diphosphate (ADP) and inorganic phosphate (Pi).
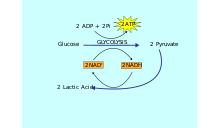
Another limitation of the lactic acid system that relates to its anaerobic quality is that only a few moles of ATP can be resynthesized from the breakdown of sugar.
[1] In activities such as running 1500 meters or a mile, the lactic acid system is used predominantly for the "kick" at the end of the race.
NADH and FADH2 are oxidized to allow the NAD+ and FAD to be reused in the aerobic system, while electrons and hydrogen ions are accepted by oxygen to produce water, a harmless byproduct.
[13] The purine nucleotide cycle is used in times of glycolytic or ATP crisis, such as strenuous exercise or starvation.
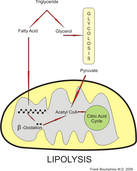
Ketones are needed as fatty acids cannot pass the blood-brain barrier, blood glucose levels are low and glycogen reserves depleted.
[15][16] After the ketones convert to acetyl-CoA in a process known as ketolysis, it enters the citric acid cycle to produce ATP by oxidative phosphorylation.
The longer that the person's glycogen reserves have been depleted, the higher the blood concentration of ketones, typically due to starvation or a low carb diet (βHB 3 - 5 mM).
Prolonged high-intensity aerobic exercise, such as running 20 miles, where individuals "hit the wall" can create post-exercise ketosis; however, the level of ketones produced are smaller (βHB 0.3 - 2 mM).
When alcohol is consumed in small quantities, the NADH/NAD+ ratio remains in balance enough for the acetyl-CoA to be used by the Krebs cycle for oxidative phosphorylation.
Without sufficient NAD+, the impaired aerobic metabolism mimics hypoxia (insufficient oxygen), resulting in excessive use of anaerobic glycolysis and a disrupted pyruvate/lactate ratio (low pyruvate, high lactate).