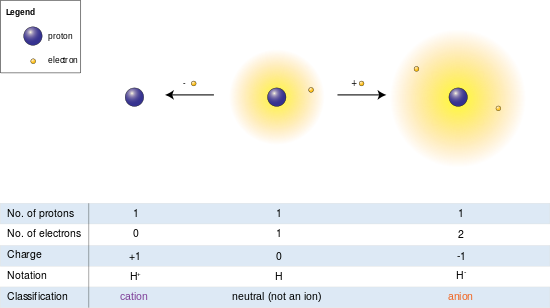
Hydrogen ion
A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particle-free space.
Hydrogen ions concentration, measured as pH, is also responsible for the acidic or basic nature of a compound.
[5] The pre-industrial state of the ocean's carbonate chemistry has been notably stable, including the balance of its pH.
[6] Following the industrial revolution, anthropogenic emissions of burning fossil fuels, cement production, and changes in land use, have increased the oceans uptake of carbon dioxide from the atmosphere by 30%.
[10] Based on Henry's Law, the amount of dissolved CO2 in an aqueous solution is directly proportional to the partial pressure of CO2 in the atmosphere.
[11] To maintain equilibrium, a state of high atmospheric partial pressure of CO2 leads to an increased oceanic exchange of this gas by molecular diffusion.
[13] The dissolving and dissociating of these inorganic carbon species generate an increase in the concentration of hydrogen ions and inversely lowers ambient surface ocean pH.
Therefore, in this model, a high concentration of the beginning reactant, carbon dioxide, produces an increased amount of end-product (H+ and CO32-), thus lowering pH and creating a more acidic solution.
[14] However, increasing atmospheric CO2 concentrations may exceed the buffering capacity threshold, consequently resulting in higher rates of ocean acidification.