Oxidative phosphorylation
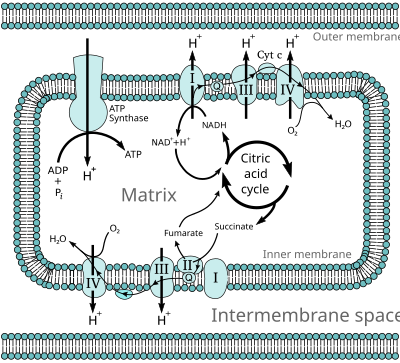
The energy stored in the chemical bonds of glucose is released by the cell in the citric acid cycle, producing carbon dioxide and the energetic electron donors NADH and FADH.
In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.
Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as superoxide and hydrogen peroxide, which lead to propagation of free radicals, damaging cells and contributing to disease and, possibly, aging and senescence.
[3] A current of protons is driven from the negative N-side of the membrane to the positive P-side through the proton-pumping enzymes of the electron transport chain.
[4] ATP synthase releases this stored energy by completing the circuit and allowing protons to flow down the electrochemical gradient, back to the N-side of the membrane.
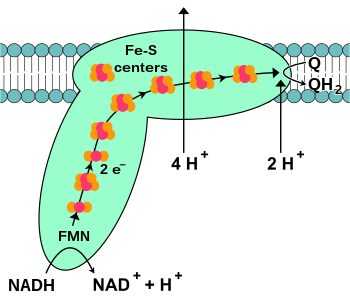
[14] This occurs by quantum tunnelling, which is rapid over distances of less than 1.4×10−9 m.[15] Many catabolic biochemical processes, such as glycolysis, the citric acid cycle, and beta oxidation, produce the reduced coenzyme NADH.
Complex II consists of four protein subunits and contains a bound flavin adenine dinucleotide (FAD) cofactor, iron–sulfur clusters, and a heme group that does not participate in electron transfer to coenzyme Q, but is believed to be important in decreasing production of reactive oxygen species.
Here, the reversed action of complex II as an oxidase is important in regenerating ubiquinol, which the parasite uses in an unusual form of pyrimidine biosynthesis.
[30] This enzyme contains a flavin and a [4Fe–4S] cluster, but, unlike the other respiratory complexes, it attaches to the surface of the membrane and does not cross the lipid bilayer.
The iron atoms inside complex III's heme groups alternate between a reduced ferrous (+2) and oxidized ferric (+3) state as the electrons are transferred through the protein.
[40] The mammalian enzyme has an extremely complicated structure and contains 13 subunits, two heme groups, as well as multiple metal ion cofactors – in all, three atoms of copper, one of magnesium and one of zinc.
The reaction catalyzed is the oxidation of cytochrome c and the reduction of oxygen: Many eukaryotic organisms have electron transport chains that differ from the much-studied mammalian enzymes described above.
However, the alternative oxidase is produced in response to stresses such as cold, reactive oxygen species, and infection by pathogens, as well as other factors that inhibit the full electron transport chain.
[54] Within such mammalian supercomplexes, some components would be present in higher amounts than others, with some data suggesting a ratio between complexes I/II/III/IV and the ATP synthase of approximately 1:1:3:7:4.
[19][56] In contrast to the general similarity in structure and function of the electron transport chains in eukaryotes, bacteria and archaea possess a large variety of electron-transfer enzymes.
[57] In common with eukaryotes, prokaryotic electron transport uses the energy released from the oxidation of a substrate to pump ions across a membrane and generate an electrochemical gradient.
(Volts) As shown above, E. coli can grow with reducing agents such as formate, hydrogen, or lactate as electron donors, and nitrate, DMSO, or oxygen as acceptors.
The small amount of energy released in this reaction is enough to pump protons and generate ATP, but not enough to produce NADH or NADPH directly for use in anabolism.
[62] This problem is solved by using a nitrite oxidoreductase to produce enough proton-motive force to run part of the electron transport chain in reverse, causing complex I to generate NADH.
Estimates of the number of protons required to synthesize one ATP have ranged from three to four,[68][69] with some suggesting cells can vary this ratio, to suit different conditions.
[67] Indeed, in the closely related vacuolar type H+-ATPases, the hydrolysis reaction is used to acidify cellular compartments, by pumping protons and hydrolysing ATP.
[76] This ATP synthesis reaction is called the binding change mechanism and involves the active site of a β subunit cycling between three states.
These reactive oxygen species and their reaction products, such as the hydroxyl radical, are very harmful to cells, as they oxidize proteins and cause mutations in DNA.
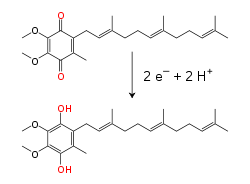
[84] Particularly important is the reduction of coenzyme Q in complex III, as a highly reactive ubisemiquinone free radical is formed as an intermediate in the Q cycle.
[94] Another advantage ectotherms tend to have in this category is an ability for their mitochondria to properly function in a wide range of temperatures, such as the western fence lizard (Sceloporus occidentalis).
However, the ray's levels were still higher than the more hypoxia-tolerant Epaulette shark (Hemiscyllum ocellatum), which potentially sees hypoxia due to the bouts of low tides that can be seen in reef platforms.
[113] This rapid respiration produces heat, and is particularly important as a way of maintaining body temperature for hibernating animals, although these proteins may also have a more general function in cells' responses to stress.
[117] Later, in 1949, Morris Friedkin and Albert L. Lehninger proved that the coenzyme NADH linked metabolic pathways such as the citric acid cycle and the synthesis of ATP.
[119][120] For another twenty years, the mechanism by which ATP is generated remained mysterious, with scientists searching for an elusive "high-energy intermediate" that would link oxidation and phosphorylation reactions.
[123][124] Subsequent research concentrated on purifying and characterizing the enzymes involved, with major contributions being made by David E. Green on the complexes of the electron-transport chain, as well as Efraim Racker on the ATP synthase.