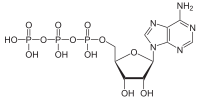
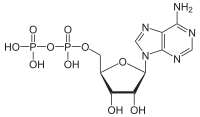
ATP hydrolysis
[1] ATP hydrolysis is the final link between the energy derived from food or sunlight and useful work such as muscle contraction, the establishment of electrochemical gradients across membranes, and biosynthetic processes necessary to maintain life.
The high negative charge density associated with the three adjacent phosphate units of ATP also destabilizes the molecule, making it higher in energy.
Hydrolysis relieves some of these electrostatic repulsions, liberating useful energy in the process by causing conformational changes in enzyme structure.
In humans, approximately 60 percent of the energy released from the hydrolysis of ATP produces metabolic heat rather than fuel the actual reactions taking place.
[5][6] The range of the ΔG value exists because this reaction is dependent on the concentration of Mg2+ cations, which stabilize the ATP molecule.
K takes into consideration reactions taking place in standard conditions, but in the cellular environment the concentrations of the involved molecules (namely, ATP, ADP, and Pi) are far from the standard 1 M. In fact, the concentrations are more appropriately measured in mM, which is smaller than M by three orders of magnitude.
[7] In one particular study, to determine ΔG in vivo in humans, the concentration of ATP, ADP, and Pi was measured using nuclear magnetic resonance.