Adenosine triphosphate
Found in all known forms of life, it is often referred to as the "molecular unit of currency" for intracellular energy transfer.
In its many reactions related to metabolism, the adenine and sugar groups remain unchanged, but the triphosphate is converted to di- and monophosphate, giving respectively the derivatives ADP and AMP.
The three phosphoryl groups are labeled as alpha (α), beta (β), and, for the terminal phosphate, gamma (γ).
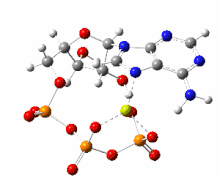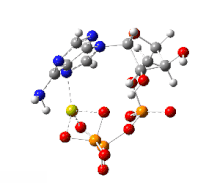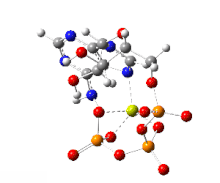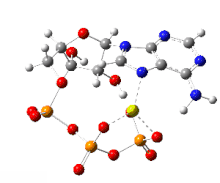
[6] Polyanionic and featuring a potentially chelating polyphosphate group, ATP binds metal cations with high affinity.
Due to the strength of the ATP-Mg2+ interaction, ATP exists in the cell mostly as a complex with Mg2+ bonded to the phosphate oxygen centers.
[15] The values of the free energy released by cleaving either a phosphate (Pi) or a pyrophosphate (PPi) unit from ATP at standard state concentrations of 1 mol/L at pH 7 are:[16] These abbreviated equations at a pH near 7 can be written more explicitly (R = adenosyl): At cytoplasmic conditions, where the ADP/ATP ratio is 10 orders of magnitude from equilibrium, the ΔG is around −57 kJ/mol.
Higher concentrations of Mg2+ decrease free energy released in the reaction due to binding of Mg2+ ions to negatively charged oxygen atoms of ATP at pH 7.
[19] ATP can be produced by a number of distinct cellular processes; the three main pathways in eukaryotes are (1) glycolysis, (2) the citric acid cycle/oxidative phosphorylation, and (3) beta-oxidation.
[20] ATP production by a non-photosynthetic aerobic eukaryote occurs mainly in the mitochondria, which comprise nearly 25% of the volume of a typical cell.
[22] A number of other small molecules can compensate for the ATP-induced shift in equilibrium conformation and reactivate PFK, including cyclic AMP, ammonium ions, inorganic phosphate, and fructose-1,6- and -2,6-biphosphate.
Although the citric acid cycle itself does not involve molecular oxygen, it is an obligately aerobic process because O2 is used to recycle the NADH and FADH2.
Although oxygen consumption appears fundamental for the maintenance of the proton motive force, in the event of oxygen shortage (hypoxia), intracellular acidosis (mediated by enhanced glycolytic rates and ATP hydrolysis), contributes to mitochondrial membrane potential and directly drives ATP synthesis.
[24] Most of the ATP synthesized in the mitochondria will be used for cellular processes in the cytosol; thus it must be exported from its site of synthesis in the mitochondrial matrix.
The citric acid cycle is regulated mainly by the availability of key substrates, particularly the ratio of NAD+ to NADH and the concentrations of calcium, inorganic phosphate, ATP, ADP, and AMP.
Each cycle of beta-oxidation shortens the fatty acid chain by two carbon atoms and produces one equivalent each of acetyl-CoA, NADH, and FADH2.
[22] An additional level of regulation is introduced by the transport rates of ATP and NADH between the mitochondrial matrix and the cytoplasm.
Ketone bodies are transported from the liver to other tissues, where acetoacetate and beta-hydroxybutyrate can be reconverted to acetyl-CoA to produce reducing equivalents (NADH and FADH2), via the citric acid cycle.
Acetoacetate in low concentrations is taken up by the liver and undergoes detoxification through the methylglyoxal pathway which ends with lactate.
The "machinery" is similar to that in mitochondria except that light energy is used to pump protons across a membrane to produce a proton-motive force.
[29] ATP is involved in signal transduction by serving as substrate for kinases, enzymes that transfer phosphate groups.
[33] This form of signal transduction is particularly important in brain function, although it is involved in the regulation of a multitude of other cellular processes.
ATP serves as a neurotransmitter in many parts of the nervous system, modulates ciliary beating, affects vascular oxygen supply etc.
When ATPase hydrolyzes the bound ATP into ADP and inorganic phosphate, myosin is positioned in a way that it can bind to actin.
[45][46] ATP has recently been proposed to act as a biological hydrotrope[47] and has been shown to affect proteome-wide solubility.
[48] Acetyl phosphate (AcP), a precursor to ATP, can readily be synthesized at modest yields from thioacetate in pH 7 and 20 °C and pH 8 and 50 °C, although acetyl phosphate is less stable in warmer temperatures and alkaline conditions than in cooler and acidic to neutral conditions.
It is unable to promote polymerization of ribonucleotides and amino acids and was only capable of phosphorylation of organic compounds.
Caution is warranted in interpreting the results of experiments using ATP analogs, since some enzymes can hydrolyze them at appreciable rates at high concentration.
[52] ATP was discovered in 1929 by Karl Lohmann [de][53] and Jendrassik[54] and, independently, by Cyrus Fiske and Yellapragada Subba Rao of Harvard Medical School,[55] both teams competing against each other to find an assay for phosphorus.
[56] It was first synthesized in the laboratory by Alexander Todd in 1948,[57] and he was awarded the Nobel Prize in Chemistry in 1957 partly for this work.
The 1978 Nobel Prize in Chemistry was awarded to Peter Dennis Mitchell for the discovery of the chemiosmotic mechanism of ATP synthesis.