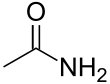
Acetamide
Yield is typically low (up to 35%), and the acetamide made this way is generated as a salt with HCl.
Notably, its dielectric constant is higher than most organic solvents, allowing it to dissolve inorganic compounds with solubilities closely analogous to that of water.
[11] Acetamide has uses in electrochemistry and the organic synthesis of pharmaceuticals, pesticides, and antioxidants for plastics.
This finding lends support to the theory that organic molecules that can lead to life (as we know it on Earth) can form in space.
On 30 July 2015, scientists reported that upon the first touchdown of the Philae lander on comet 67/P's surface, measurements by the COSAC and Ptolemy instruments revealed sixteen organic compounds, four of which – acetamide, acetone, methyl isocyanate, and propionaldehyde[15][16][17] – were seen for the first time on a comet.