Hydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force.
[6] The most frequent donor and acceptor atoms are nitrogen (N), oxygen (O), and fluorine (F), due to their high electronegativity and ability to engage in stronger hydrogen bonding.
The term "hydrogen bond" is generally used for well-defined, localized interactions with significant charge transfer and orbital overlap, such as those in DNA base pairing or ice.
It is responsible for the anomalously high boiling point of water, the stabilization of protein and nucleic acid structures, and key properties of materials like paper, wool, and hydrogels.
In biological systems, hydrogen bonds mediate molecular recognition, enzyme catalysis, and DNA replication, while in materials science, they contribute to self-assembly, adhesion, and supramolecular organization.
[citation needed] Hydrogen bonds arise from a combination of electrostatics (multipole-multipole and multipole-induced multipole interactions), covalency (charge transfer by orbital overlap), and dispersion (London forces).
Although weak (≈1 kcal/mol), "non-traditional" hydrogen bonding interactions are ubiquitous and influence structures of many kinds of materials.
[citation needed] The definition of hydrogen bonding has gradually broadened over time to include these weaker attractive interactions.
[17][18] Typical enthalpies in vapor include:[19] The strength of intermolecular hydrogen bonds is most often evaluated by measurements of equilibria between molecules containing donor and/or acceptor units, most often in solution.
Structural details, in particular distances between donor and acceptor which are smaller than the sum of the van der Waals radii can be taken as indication of the hydrogen bond strength.
This description of the hydrogen bond has been proposed to describe unusually short distances generally observed between O=C−OH··· or ···O=C−C=C−OH.
The amide I mode of backbone carbonyls in α-helices shifts to lower frequencies when they form H-bonds with side-chain hydroxyl groups.
[28] In the hydrogen bonding network in protic organic ionic plastic crystals (POIPCs), which are a type of phase change material exhibiting solid-solid phase transitions prior to melting, variable-temperature infrared spectroscopy can reveal the temperature dependence of hydrogen bonds and the dynamics of both the anions and the cations.
[29] The sudden weakening of hydrogen bonds during the solid-solid phase transition seems to be coupled with the onset of orientational or rotational disorder of the ions.
[30] According to a modern description O:H−O integrates both the intermolecular O:H lone pair ":" nonbond and the intramolecular H−O polar-covalent bond associated with O−O repulsive coupling.
[citation needed] Interpretations of the anisotropies in the Compton profile of ordinary ice claim that the hydrogen bond is partly covalent.
However, hydrogen bonding is generally still a bound state phenomenon, since the interaction energy has a net negative sum.
This interpretation remained controversial until NMR techniques demonstrated information transfer between hydrogen-bonded nuclei, a feat that would only be possible if the hydrogen bond contained some covalent character.
[40] In that paper, Latimer and Rodebush cited the work of a fellow scientist at their laboratory, Maurice Loyal Huggins, saying, "Mr. Huggins of this laboratory in some work as yet unpublished, has used the idea of a hydrogen kernel held between two atoms as a theory in regard to certain organic compounds."
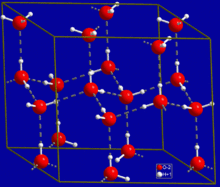
Hydrogen bonding strongly affects the crystal structure of ice, helping to create an open hexagonal lattice.
Hydrogen bonding plays an important role in determining the three-dimensional structures and the properties adopted by many proteins.
Thus, hydrogen bonds can be broken by chemical or mechanical means while retaining the basic structure of the polymer backbone.
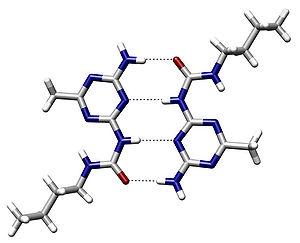
For example, the double helical structure of DNA is due largely to hydrogen bonding between its base pairs (as well as pi stacking interactions), which link one complementary strand to the other and enable replication.
When the spacing of the amino acid residues participating in a hydrogen bond occurs regularly between positions i and i + 4, an alpha helix is formed.
Hydrogen bonds also play a part in forming the tertiary structure of protein through interaction of R-groups.
[51] Several studies have shown that hydrogen bonds play an important role for the stability between subunits in multimeric proteins.
The exogenous dehydration enhances the electrostatic interaction between the amide and carbonyl groups by de-shielding their partial charges.
In nylon, hydrogen bonds between carbonyl and the amide NH effectively link adjacent chains, which gives the material mechanical strength.
Generally, the hydrogen bond is characterized by a proton acceptor that is a lone pair of electrons in nonmetallic atoms (most notably in the nitrogen, and chalcogen groups).