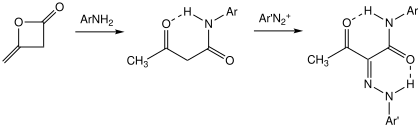
Acetoacetanilide
This chemical and many related compounds (prepared from various aniline derivatives) are used in the production of organic pigments called arylide yellows, one example being Pigment Yellow 74.
Acetoacetanilide crystallizes as the keto-amide tautomer according to X-ray crystallography.
The molecules are linked by intermolecular hydrogen bonds, which allows the benzoyl ketone to rotate out of the plane of the amide.
[1] For the general case of substituted acetoanilides, substituents on the aryl ring affect the balance of intra- vs intermolecular hydrogen bonding.
[6] In the presence of sulfuric acid, acetoacetanilide dehydrates to give 4-methyl-2-quinolone.