Adamantane
This similarity led to the name adamantane, which is derived from the Greek adamantinos (relating to steel or diamond).
The discovery of adamantane in petroleum in 1933 launched a new field of chemistry dedicated to the synthesis and properties of polyhedral organic compounds.
Adamantane derivatives have found practical application as drugs, polymeric materials, and thermally stable lubricants.
[5] The first attempted laboratory synthesis was made in 1924 by German chemist Hans Meerwein using the reaction of formaldehyde with diethyl malonate in the presence of piperidine.
[11][12] The process was still too complex, and a more convenient method was found in 1957 by Paul von Ragué Schleyer: dicyclopentadiene was first hydrogenated in the presence of a catalyst (e.g. platinum dioxide) to give tricyclodecane and then transformed into adamantane using a Lewis acid (e.g. aluminium chloride) as another catalyst.
Melt growth results in the worst crystalline quality with a mosaic spread in the X-ray reflection of about 1°.
Slow cooling of the tube, while maintaining the temperature gradient, gradually shifts the melting zone (rate ~2 mm/hour), producing a single-crystal boule.
[8] Petroleum remains a source of adamantane; the content varies from between 0.0001% and 0.03% depending on the oil field and is too low for commercial production.
[21] Their isolation from a complex mixture of hydrocarbons is possible due to their high melting point and the ability to distill with water vapor and form stable adducts with thiourea.
In particular, whereas adamantane molecules freely rotate in the cubic phase, they are frozen in the tetragonal one; the density increases stepwise from 1.08 to 1.18 g/cm3, and the entropy changes by a significant amount of 1594 J/(mol·K).
[17] Elastic constants of adamantane were measured using large (centimeter-sized) single crystals and the ultrasonic echo technique.
[17][18][27] The nuclear magnetic resonance (NMR) spectrum of adamantane consists of two poorly resolved signals, which correspond to sites 1 and 2 (see picture below).
[3][28] The infrared absorption spectrum of adamantane is relatively simple because of the high symmetry of the molecule.
The functional group derived from adamantane is adamantyl, formally named as 1-adamantyl or 2-adamantyl depending on which site is connected to the parent molecule.
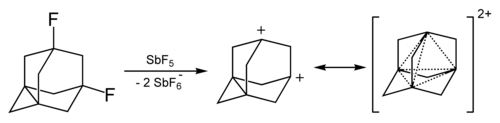
[43] In all these cases, reaction proceeded via formation of the adamantane cation which then interacted with fluorinated nucleophiles.
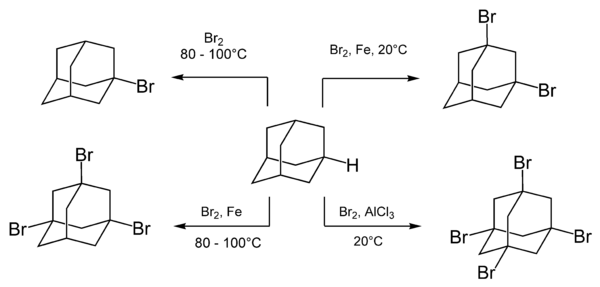
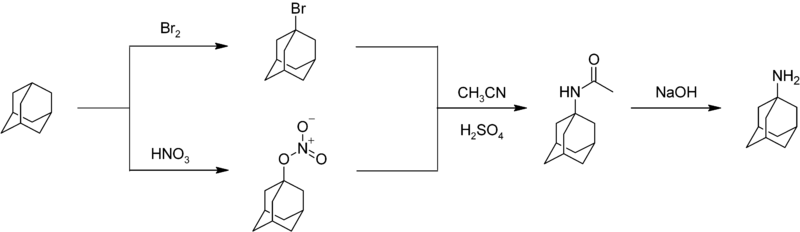
[48] A nitrogen-substituted drug amantadine can be prepared by reacting adamantane with bromine or nitric acid to give the bromide or nitroester at the 1-position.
Reaction of either compound with acetonitrile affords the acetamide, which is hydrolyzed to give 1-adamantylamine:[49] Adamantane itself enjoys few applications since it is merely an unfunctionalized hydrocarbon.
[51] In dye lasers, adamantane may be used to extend the life of the gain medium; it cannot be photoionized under atmosphere because its absorption bands lie in the vacuum-ultraviolet region of the spectrum.
[54][55] Other drugs among adamantane derivatives include adapalene, adapromine, bromantane (bromantan), carmantadine, chlodantane (chlodantan), dopamantine, gludantan (gludantane), hemantane (hymantane), idramantone (kemantane), memantine, nitromemantine rimantadine, saxagliptin, somantadine, tromantadine, and vildagliptin.
Adamantane was recently identified as a key structural subunit in several synthetic cannabinoid designer drugs, namely AB-001 and SDB-001.
[57] Adamantane is an attractive candidate for propellant in Hall-effect thrusters because it ionizes easily, can be stored in solid form rather than a heavy pressure tank, and is relatively nontoxic.
[59] Adamantane-based polymers might find application for coatings of touchscreens,[60] and there are prospects for using adamantane and its homologues in nanotechnology.
For example, the soft cage-like structure of adamantane solid allows incorporation of guest molecules, which can be released inside the human body upon breaking the matrix.
[64] Particularly notorious is tetramethylenedisulfotetramine, often shortened to "tetramine", a rodenticide banned in most countries for extreme toxicity to humans.
[65] Arsenicin A is a naturally occurring organoarsenic chemical isolated from the New Caledonian sea sponge Echinochalina bargibanti and is the first known heterocycle to contain multiple arsenic atoms.