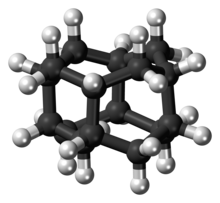
Diamantane
It is a colorless solid that has been a topic of research since its discovery in oil and separation from deep natural gas condensates.
Although present in only trace concentrations in typical oils, due to their great thermodynamic stability, diamondoids such as diamantane are naturally concentrated by catagenesis, becoming important constituents of some natural gas condensates including those from the Norphlet Formation, U.S. Gulf of Mexico, and the Western Canada Basin.
[2] Diamantane was chosen as the Congress Emblem of the 1963 London IUPAC meeting, and was featured as a decoration on the cover of abstracts, program, and publicity material.
[3] The year 1966 also marked the isolation of diamantane from the high-boiling fractions of the crude oil of Hodonin (from which adamantane was discovered) and the achievement of a significant improvement in its yield (to 10%).
[3] The convenient, synthetic route begins with the dimerization of norbornadiene (1) catalyzed by a mixture of cobalt bromide-triphenylphosphine and boron trifluoride etherate.
The next step is a rearrangement, which occurs in a hot solution of cyclohexane or carbon disulfide with aluminum bromide and forms the main product diamantane (4).
Diamantane can be nitrated by treatment with nitronium tetrafluoroborate (in nitrile-free nitromethane) to give a mixture of two isomeric nitrodiamantanes.