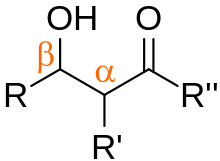Aldol
In organic chemistry, an aldol is a structure consisting of a hydroxy group (-OH) two carbons away from either an aldehyde or a ketone.
The name combines the suffix 'ol' from the alcohol and the prefix depending on the carbonyl group, either 'ald' for an aldehyde, or 'ket' for a ketone, in which case it referred to as a 'ketol'.
[1][2] Aldols are the product of a carbon-carbon bond-formation reaction, giving them wide applicability as a precursor for a variety of other compounds.
[1] These reactions may also be done intramolecularly to form 5 or 6 member rings or for stereoselective syntheses in the active area of asymmetric synthesis.
Aldols may also undergo a condensation reaction in which the hydroxy group is replaced by a pi bond.