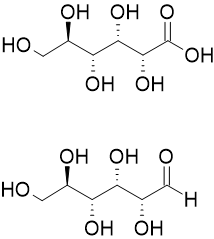
Aldonic acid
However, these rings do not have a chiral carbon at the terminal end bearing the aldehyde, and they cannot form R−O−R′ linkages between different molecules.
Aldonic acids are most commonly prepared by the oxidation of the sugar with bromine and water under neutral pH.
In commercial settings, glucose, galactose, or arabinose are commonly oxidized to obtain aldonic acids.
Aldonic acids can be used as the natural starting materials to synthetic products[9] including polyesters and polyurethane.
[11] Aldonic acids are typically used in industrial applications for their ability to degrade naturally in the environment.