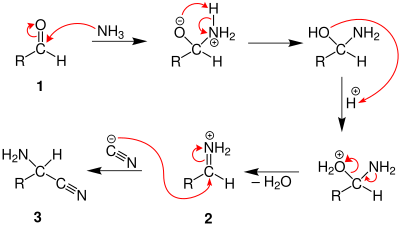
Strecker amino acid synthesis
The condensation reaction yields an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino acid.
Likewise, the usage of ketones, instead of aldehydes, gives α,α-disubstituted amino acids.
[4] In the first part of the reaction process, the carbonyl is converted to an iminium, to which a cyanide ion adds.
[8] The German chemist Adolph Strecker discovered the series of chemical reactions that produce an amino acid from an aldehyde or ketone.
In the original Strecker reaction acetaldehyde, ammonia, and hydrogen cyanide combined to form after hydrolysis alanine.
Using primary and secondary amines in place of ammonium was shown to yield N-substituted amino acids.