Alkali metal
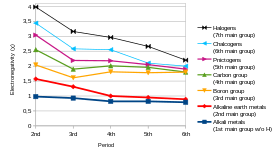
[note 4] Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour.
[19] Berzelius gave the unknown material the name lithion/lithina, from the Greek word λιθoς (transliterated as lithos, meaning "stone"), to reflect its discovery in a solid mineral, as opposed to potassium, which had been discovered in plant ashes, and sodium, which was known partly for its high abundance in animal blood.
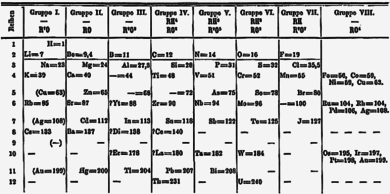
[20][15][18] Lithium, sodium, and potassium were part of the discovery of periodicity, as they are among a series of triads of elements in the same group that were noted by Johann Wolfgang Döbereiner in 1850 as having similar properties.
[21] After 1869, Dmitri Mendeleev proposed his periodic table placing lithium at the top of a group with sodium, potassium, rubidium, caesium, and thallium.
[5] There were at least four erroneous and incomplete discoveries[30][31][32][33] before Marguerite Perey of the Curie Institute in Paris, France discovered francium in 1939 by purifying a sample of actinium-227, which had been reported to have a decay energy of 220 keV.
The new product exhibited chemical properties of an alkali metal (such as coprecipitating with caesium salts), which led Perey to believe that it was element 87, caused by the alpha decay of actinium-227.
[37][38] It is highly unlikely[37] that this reaction will be able to create any atoms of ununennium in the near future, given the extremely difficult task of making sufficient amounts of einsteinium-254, which is favoured for production of ultraheavy elements because of its large mass, relatively long half-life of 270 days, and availability in significant amounts of several micrograms,[39] to make a large enough target to increase the sensitivity of the experiment to the required level; einsteinium has not been found in nature and has only been produced in laboratories, and in quantities smaller than those needed for effective synthesis of superheavy elements.
[41][42] No attempts at synthesis have been made for any heavier alkali metals: due to their extremely high atomic number, they would require new, more powerful methods and technology to make.
Lithium is also much less abundant than sodium and potassium as it is poorly synthesised in both Big Bang nucleosynthesis and in stars: the Big Bang could only produce trace quantities of lithium, beryllium and boron due to the absence of a stable nucleus with 5 or 8 nucleons, and stellar nucleosynthesis could only pass this bottleneck by the triple-alpha process, fusing three helium nuclei to form carbon, and skipping over those three elements.
Due to planetary differentiation, the core region is believed to be primarily composed of iron (88.8%), with smaller amounts of nickel (5.8%), sulfur (4.5%), and less than 1% trace elements.
[10]: 74 Additionally, the heavy alkaline earth metals calcium, strontium, and barium, as well as the divalent lanthanides europium and ytterbium, are pale yellow, though the colour is much less prominent than it is for caesium.
[73] They react aggressively with the halogens to form the alkali metal halides, which are white ionic crystalline compounds that are all soluble in water except lithium fluoride (LiF).
[5] Not only do the alkali metals react with water, but also with proton donors like alcohols and phenols, gaseous ammonia, and alkynes, the last demonstrating the phenomenal degree of their reactivity.
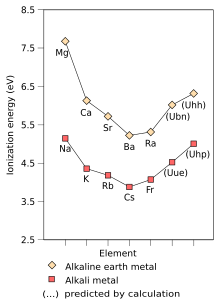
[10]: 76 The second ionisation energy of all of the alkali metals is very high[5][64] as it is in a full shell that is also closer to the nucleus;[5] thus, they almost always lose a single electron, forming cations.
[10]: 76 Francium is also predicted to show some differences due to its high atomic weight, causing its electrons to travel at considerable fractions of the speed of light and thus making relativistic effects more prominent.
[97] Its chemical properties as one of the alkali metals make it one of the most problematic of the short-to-medium-lifetime fission products because it easily moves and spreads in nature due to the high water solubility of its salts, and is taken up by the body, which mistakes it for its essential congeners sodium and potassium.
[10]: 87 The alkali metals form many intermetallic compounds with each other and the elements from groups 2 to 13 in the periodic table of varying stoichiometries,[10]: 81 such as the sodium amalgams with mercury, including Na5Hg8 and Na3Hg.
[10]: 285 When the alkali metals react with the heavier elements in the carbon group (silicon, germanium, tin, and lead), ionic substances with cage-like structures are formed, such as the silicides M4Si4 (M = K, Rb, or Cs), which contains M+ and tetrahedral Si4−4 ions.
[10]: 417 However, sodium and potassium form colourless azide salts involving the linear N−3 anion; due to the large size of the alkali metal cations, they are thermally stable enough to be able to melt before decomposing.
[10]: 76 All twenty stable alkali metal halides are known; the unstable ones are not known, with the exception of sodium astatide, because of the great instability and rarity of astatine and francium.
Organolithium compounds are electrically non-conducting volatile solids or liquids that melt at low temperatures, and tend to form oligomers with the structure (RLi)x where R is the organic group.
As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful bases and nucleophiles.
[65][140] Organolithium compounds, especially n-butyllithium, are useful reagents in organic synthesis, as might be expected given lithium's diagonal relationship with magnesium, which plays an important role in the Grignard reaction.
They thermally decompose to eliminate a β-hydrogen, producing alkenes and lithium hydride: another route is the reaction of ethers with alkyl- and aryllithiums that act as strong bases.
[10]: 105 In non-polar solvents, aryllithiums react as the carbanions they effectively are, turning carbon dioxide to aromatic carboxylic acids (ArCO2H) and aryl ketones to tertiary carbinols (Ar'2C(Ar)OH).
[122]: 256 Like the alkali metals, cobaltocene is a strong reducing agent, and decamethylcobaltocene is stronger still due to the combined inductive effect of the ten methyl groups.
[5] However, these methods are problematic because the potassium metal tends to dissolve in its molten chloride and vaporises significantly at the operating temperatures, potentially forming the explosive superoxide.
[215] Studies on the light emitted by laser-trapped francium-210 ions have provided accurate data on transitions between atomic energy levels, similar to those predicted by quantum theory.
[216] Pure alkali metals are dangerously reactive with air and water and must be kept away from heat, fire, oxidising agents, acids, most organic compounds, halocarbons, plastics, and moisture.
[240] There is only very limited evidence in the form of deficiency symptoms for rubidium being possibly essential in goats; even if this is true, the trace amounts usually present in food are more than enough.